This is a preprint.
Dissection of medical AI reasoning processes via physician and generative-AI collaboration
- PMID: 37292705
- PMCID: PMC10246034
- DOI: 10.1101/2023.05.12.23289878
Dissection of medical AI reasoning processes via physician and generative-AI collaboration
Abstract
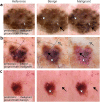
Despite the proliferation and clinical deployment of artificial intelligence (AI)-based medical software devices, most remain black boxes that are uninterpretable to key stakeholders including patients, physicians, and even the developers of the devices. Here, we present a general model auditing framework that combines insights from medical experts with a highly expressive form of explainable AI that leverages generative models, to understand the reasoning processes of AI devices. We then apply this framework to generate the first thorough, medically interpretable picture of the reasoning processes of machine-learning-based medical image AI. In our synergistic framework, a generative model first renders "counterfactual" medical images, which in essence visually represent the reasoning process of a medical AI device, and then physicians translate these counterfactual images to medically meaningful features. As our use case, we audit five high-profile AI devices in dermatology, an area of particular interest since dermatology AI devices are beginning to achieve deployment globally. We reveal how dermatology AI devices rely both on features used by human dermatologists, such as lesional pigmentation patterns, as well as multiple, previously unreported, potentially undesirable features, such as background skin texture and image color balance. Our study also sets a precedent for the rigorous application of explainable AI to understand AI in any specialized domain and provides a means for practitioners, clinicians, and regulators to uncloak AI's powerful but previously enigmatic reasoning processes in a medically understandable way.
Conflict of interest statement
R.D. reports fees from L’Oreal, Frazier Healthcare Partners, Pfizer, DWA, and VisualDx for consulting; stock options from MDAcne and Revea for advisory board; and research funding from UCB.
Figures



Similar articles
-
Auditing the inference processes of medical-image classifiers by leveraging generative AI and the expertise of physicians.Nat Biomed Eng. 2025 Mar;9(3):294-306. doi: 10.1038/s41551-023-01160-9. Epub 2023 Dec 28. Nat Biomed Eng. 2025. PMID: 38155295
-
GANterfactual-Counterfactual Explanations for Medical Non-experts Using Generative Adversarial Learning.Front Artif Intell. 2022 Apr 8;5:825565. doi: 10.3389/frai.2022.825565. eCollection 2022. Front Artif Intell. 2022. PMID: 35464995 Free PMC article.
-
A domain knowledge-based interpretable deep learning system for improving clinical breast ultrasound diagnosis.Commun Med (Lond). 2024 May 17;4(1):90. doi: 10.1038/s43856-024-00518-7. Commun Med (Lond). 2024. PMID: 38760506 Free PMC article.
-
Artificial intelligence in molecular imaging.Ann Transl Med. 2021 May;9(9):824. doi: 10.21037/atm-20-6191. Ann Transl Med. 2021. PMID: 34268437 Free PMC article. Review.
-
Explainable AI for Bioinformatics: Methods, Tools and Applications.Brief Bioinform. 2023 Sep 20;24(5):bbad236. doi: 10.1093/bib/bbad236. Brief Bioinform. 2023. PMID: 37478371 Review.
References
-
- Wu E. et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nature Medicine 27, 582–584 (2021). - PubMed
-
- Reddy S. Explainability and artificial intelligence in medicine. The Lancet Digital Health 4, E214–E215 (4 2022). - PubMed
-
- DeGrave A. J., Janizek J. D. & Lee S.-I. AI for radiographic COVID-19 detection selects shortcuts over signal. Nature Machine Intelligence (2021).
-
- Singh N. et al. Agreement between saliency maps and human-labeled regions of interest: applications to skin disease classification (2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
