This is a preprint.
Malaria Transmission Intensity and Parasitemia during the Three-Dose RTS,S/AS01 Vaccination Series do not Reduce Magnitude of Antibody Response nor Efficacy Against the First Case of Malaria
- PMID: 37292711
- PMCID: PMC10246269
- DOI: 10.21203/rs.3.rs-2960373/v1
Malaria Transmission Intensity and Parasitemia during the Three-Dose RTS,S/AS01 Vaccination Series do not Reduce Magnitude of Antibody Response nor Efficacy Against the First Case of Malaria
Update in
-
Background malaria incidence and parasitemia during the three-dose RTS,S/AS01 vaccination series do not reduce magnitude of antibody response nor efficacy against the first case of malaria.BMC Infect Dis. 2023 Oct 23;23(1):716. doi: 10.1186/s12879-023-08699-7. BMC Infect Dis. 2023. PMID: 37872492 Free PMC article.
Abstract
Background: RTS,S/AS01 has been recommended by WHO for widespread implementation in medium to high malaria transmission settings. Previous analyses have noted lower vaccine efficacies in higher transmission settings, possibly due to the more rapid development of naturally acquired immunity in the control group.
Methods: To investigate a reduced immune response to vaccination as a potential mechanism behind lower efficacy in high transmission areas, we examine initial vaccine antibody (anti-CSP IgG) response and vaccine efficacy against the first case of malaria to exclude the delayed malaria effect using data from three study areas (Kintampo, Ghana; Lilongwe, Malawi; Lambaréné, Gabon) from the 2009-2014 phase III trial (NCT00866619). Our key exposures are parasitemia during the vaccination series and malaria transmission intensity. We calculate vaccine efficacy (one minus hazard ratio) using a cox-proportional hazards model and allowing for the time-varying effect of RTS,S/AS01.
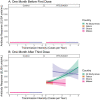
Results: We find that antibody responses to the primary three-dose vaccination series were higher in Ghana than in Malawi and Gabon, but that neither antibody levels nor vaccine efficacy against the first case of malaria varied by transmission intensity or parasitemia during the primary vaccination series.
Conclusions: We find that vaccine efficacy is unrelated to infections during vaccination. Contributing to a conflicting literature, our results suggest that vaccine efficacy is also unrelated to infections before vaccination, meaning that delayed malaria is likely the main reason for lower efficacy in high transmission settings, not reduced immune responses. This may be reassuring for implementation in high transmission settings, though further studies are needed.
Keywords: Africa; GIS; delayed malaria; geographic information system; immunology; rebound malaria; vaccine.
Conflict of interest statement
Conflicts of Interest The authors declare no conflicts of interest. GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation.
Figures


References
-
- Organization WH, Others. World malaria report 2021. 2021; Available from: https://apps.who.int/iris/bitstream/handle/10665/350147/9789240040496-en...
-
- Bell GJ, Agnandji ST, Asante KP, Ghansah A, Kamthunzi P, Emch M, et al. Impacts of Ecology, Parasite Antigenic Variation, and Human Genetics on RTS,S/AS01e Malaria Vaccine Efficacy. Current Epidemiology Reports [Internet]. 2021. Jul 30; Available from: 10.1007/s40471-021-00271-8 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

