This is a preprint.
Parallelized immunomagnetic nanopore sorting: modeling, scaling, and optimization of surface marker specific isolation of extracellular vesicles from complex media
- PMID: 37292737
- PMCID: PMC10246262
- DOI: 10.21203/rs.3.rs-2913647/v1
Parallelized immunomagnetic nanopore sorting: modeling, scaling, and optimization of surface marker specific isolation of extracellular vesicles from complex media
Update in
-
Modeling and optimization of parallelized immunomagnetic nanopore sorting for surface marker specific isolation of extracellular vesicles from complex media.Sci Rep. 2023 Aug 16;13(1):13292. doi: 10.1038/s41598-023-39746-7. Sci Rep. 2023. PMID: 37587235 Free PMC article.
Abstract
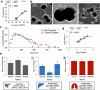
The isolation of specific subpopulations of extracellular vesicles (EVs) based on their expression of surface markers poses a significant challenge due to their nanoscale size (< 800 nm), their heterogeneous surface marker expression, and the vast number of background EVs present in clinical specimens (10 10 -10 12 EVs/mL in blood). Highly parallelized nanomagnetic sorting using track etched magnetic nanopore (TENPO) chips has achieved precise immunospecific sorting with high throughput and resilience to clogging. However, there has not yet been a systematic study of the design parameters that control the trade-offs in throughput, target EV recovery, and specificity in this approach. We combine finite-element simulation and experimental characterization of TENPO chips to elucidate design rules to isolate EV subpopulations from blood. We demonstrate the utility of this approach by increasing specificity > 10x relative to prior published designs without sacrificing recovery of the target EVs by selecting pore diameter, number of membranes placed in series, and flow rate. We compare TENPO-isolated EVs to those of gold-standard methods of EV isolation and demonstrate its utility for wide application and modularity by targeting subpopulations of EVs from multiple models of disease including lung cancer, pancreatic cancer, and liver cancer.
Conflict of interest statement
Conflicts of Interest
For our conflicts of interest to disclose, Dr. David Issadore is a founder of Chip Diagnostics and holds equity in the company. The other authors listed do not have competing interests.
Figures



References
-
- Lin A. A., Nimgaonkar V., Issadore D., & Carpenter E. L. Extracellular Vesicle–Based Multianalyte Liquid Biopsy as a Diagnostic for Cancer. Ann. Rev. of Biomed. Data Sci, 5. (2022). - PubMed
-
- Ko J., et al. Combining machine learning and nanofluidic technology to diagnose pancreatic cancer using exosomes. ACS Nano, 11(11), 11182–11193. (2017). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

