This is a preprint.
Higher Neuronal Facilitation and Potentiation with APOE4 Suppressed by Angiotensin II
- PMID: 37292788
- PMCID: PMC10246245
- DOI: 10.21203/rs.3.rs-2960437/v1
Higher Neuronal Facilitation and Potentiation with APOE4 Suppressed by Angiotensin II
Update in
-
Higher Neuronal Facilitation and Potentiation with APOE4 Suppressed by Angiotensin II.Mol Neurobiol. 2024 Jan;61(1):120-131. doi: 10.1007/s12035-023-03556-9. Epub 2023 Aug 17. Mol Neurobiol. 2024. PMID: 37589833 Free PMC article.
Abstract
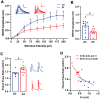
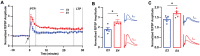
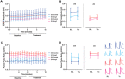
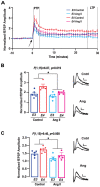
Progressive hippocampal degeneration is a key component of Alzheimer's disease (AD) progression. Therefore, identifying how hippocampal neuronal function is modulated early in AD is an important approach to eventually prevent degeneration. AD-risk factors and signaling molecules likely modulate neuronal function, including APOE genotype and angiotensin II. Compared to APOE3 , APOE4 increases AD risk up to 12-fold, and high levels of angiotensin II are hypothesized to disrupt neuronal function in AD. However, the extent that APOE and angiotensin II modulates the hippocampal neuronal phenotype in AD-relevant models is unknown. To address this issue, we used electrophysiological techniques to assess the impact of APOE genotype and angiotensin II on basal synaptic transmission, presynaptic and post-synaptic activity in mice that express human APOE3 (E3FAD) or APOE4 (E4FAD) and overproduce Aβ. We found that compared to E3FAD mice, E4FAD mice had lower basal synaptic activity, but higher levels of paired pulse facilitation (PPF) and Long-Term Potentiation (LTP) in the Schaffer Collateral Commissural Pathway (SCCP) of the hippocampus. We also found that exogenous angiotensin II has a profound inhibitory effect on hippocampal LTP in both E3FAD and E4FAD mice. Collectively, our data suggests that APOE4 and Aβ are associated with a hippocampal phenotype comprised of lower basal activity and higher responses to high frequency stimulation, the latter of which is suppressed by angiotensin II. These novel data suggest a potential mechanistic link between hippocampal activity, APOE4 genotype and angiotensin II in AD.
Conflict of interest statement
Figures
Similar articles
-
Higher Neuronal Facilitation and Potentiation with APOE4 Suppressed by Angiotensin II.Mol Neurobiol. 2024 Jan;61(1):120-131. doi: 10.1007/s12035-023-03556-9. Epub 2023 Aug 17. Mol Neurobiol. 2024. PMID: 37589833 Free PMC article.
-
APOE genotype-dependent pharmacogenetic responses to rapamycin for preventing Alzheimer's disease.Neurobiol Dis. 2020 Jun;139:104834. doi: 10.1016/j.nbd.2020.104834. Epub 2020 Mar 12. Neurobiol Dis. 2020. PMID: 32173556 Free PMC article. Review.
-
APOE4 enhances age-dependent decline in cognitive function by down-regulating an NMDA receptor pathway in EFAD-Tg mice.Mol Neurodegener. 2015 Mar 5;10:7. doi: 10.1186/s13024-015-0002-2. Mol Neurodegener. 2015. PMID: 25871877 Free PMC article.
-
Systemic Candesartan Treatment Modulates Behavior, Synaptic Protein Levels, and Neuroinflammation in Female Mice That Express Human APOE4.Front Neurosci. 2021 Feb 10;15:628403. doi: 10.3389/fnins.2021.628403. eCollection 2021. Front Neurosci. 2021. PMID: 33642985 Free PMC article.
-
APOE-modulated Aβ-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective.J Neurochem. 2015 May;133(4):465-88. doi: 10.1111/jnc.13072. Epub 2015 Mar 18. J Neurochem. 2015. PMID: 25689586 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

