This is a preprint.
Tumor Cytokine-Induced Hepatic Gluconeogenesis Contributes to Cancer Cachexia: Insights from Full Body Single Nuclei Sequencing
- PMID: 37292804
- PMCID: PMC10245574
- DOI: 10.1101/2023.05.15.540823
Tumor Cytokine-Induced Hepatic Gluconeogenesis Contributes to Cancer Cachexia: Insights from Full Body Single Nuclei Sequencing
Abstract
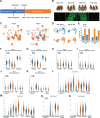
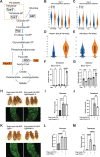
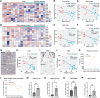
A primary cause of death in cancer patients is cachexia, a wasting syndrome attributed to tumor-induced metabolic dysregulation. Despite the major impact of cachexia on the treatment, quality of life, and survival of cancer patients, relatively little is known about the underlying pathogenic mechanisms. Hyperglycemia detected in glucose tolerance test is one of the earliest metabolic abnormalities observed in cancer patients; however, the pathogenesis by which tumors influence blood sugar levels remains poorly understood. Here, utilizing a Drosophila model, we demonstrate that the tumor secreted interleukin-like cytokine Upd3 induces fat body expression of Pepck1 and Pdk, two key regulatory enzymes of gluconeogenesis, contributing to hyperglycemia. Our data further indicate a conserved regulation of these genes by IL-6/JAK-STAT signaling in mouse models. Importantly, in both fly and mouse cancer cachexia models, elevated gluconeogenesis gene levels are associated with poor prognosis. Altogether, our study uncovers a conserved role of Upd3/IL-6/JAK-STAT signaling in inducing tumor-associated hyperglycemia, which provides insights into the pathogenesis of IL-6 signaling in cancer cachexia.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
