This is a preprint.
Idiopathic subglottic stenosis arises at the epithelial interface of host and pathogen
- PMID: 37292825
- PMCID: PMC10246274
- DOI: 10.21203/rs.3.rs-2945067/v1
Idiopathic subglottic stenosis arises at the epithelial interface of host and pathogen
Update in
-
Idiopathic Subglottic Stenosis and the Epithelial Interface of Host and Environment.J Am Coll Surg. 2025 Aug 1;241(2):180-192. doi: 10.1097/XCS.0000000000001340. Epub 2025 Jul 16. J Am Coll Surg. 2025. PMID: 39902917
Abstract
Background: Idiopathic subglottic stenosis (iSGS) is a rare fibrotic disease of the proximal airway affecting adult Caucasian women nearly exclusively. Life-threatening ventilatory obstruction occurs secondary to pernicious subglottic mucosal scar. Disease rarity and wide geographic patient distribution has previously limited substantive mechanistic investigation into iSGS pathogenesis.
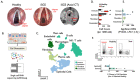
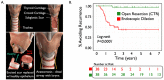
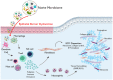
Result: By harnessing pathogenic mucosa from an international iSGS patient cohort and single-cell RNA sequencing, we unbiasedly characterize the cell subsets in the proximal airway scar and detail their molecular phenotypes. Results show that the airway epithelium in iSGS patients is depleted of basal progenitor cells, and the residual epithelial cells acquire a mesenchymal phenotype. Observed displacement of bacteria beneath the lamina propria provides functional support for the molecular evidence of epithelial dysfunction. Matched tissue microbiomes support displacement of the native microbiome into the lamina propria of iSGS patients rather than disrupted bacterial community structure. However, animal models confirm that bacteria are necessary for pathologic proximal airway fibrosis and suggest an equally essential role for host adaptive immunity. Human samples from iSGS airway scar demonstrate adaptive immune activation in response to the proximal airway microbiome of both matched iSGS patients and healthy controls. Clinical outcome data from iSGS patients suggests surgical extirpation of airway scar and reconstitution with unaffected tracheal mucosa halts the progressive fibrosis.
Conclusion: Our data support an iSGS disease model where epithelial alterations facilitate microbiome displacement, dysregulated immune activation, and localized fibrosis. These results refine our understanding of iSGS and implicate shared pathogenic mechanisms with distal airway fibrotic diseases.
Conflict of interest statement
COMPETING INTERESTS The authors of this manuscript declare no financial or other conflicts of interest to disclose as described by the journal Microbiome.
Figures



References
-
- Bachmaier K., Neu N., de la Maza L.M., Pal S., Hessel A., and Penninger J.M. (1999). Chlamydia infections and heart disease linked through antigenic mimicry. Science 283, 1335–1339. - PubMed
-
- Daniero J.J., Ekbom D.C., Gelbard A., Akst L.M., and Hillel A.T. (2017). Inaugural Symposium on Advanced Surgical Techniques in Adult Airway Reconstruction: Proceedings of the North American Airway Collaborative (NoAAC). JAMA Otolaryngol Head Neck Surg 143, 609–613. 10.1001/jamaoto.2016.4126. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

