This is a preprint.
Disrupted memory T cell expansion in HIV-exposed uninfected infants is preceded by premature skewing of T cell receptor clonality
- PMID: 37292866
- PMCID: PMC10245741
- DOI: 10.1101/2023.05.19.540713
Disrupted memory T cell expansion in HIV-exposed uninfected infants is preceded by premature skewing of T cell receptor clonality
Update in
-
Premature skewing of T cell receptor clonality and delayed memory expansion in HIV-exposed infants.Nat Commun. 2024 May 14;15(1):4080. doi: 10.1038/s41467-024-47955-5. Nat Commun. 2024. PMID: 38744812 Free PMC article.
Abstract
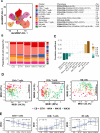
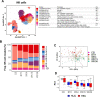
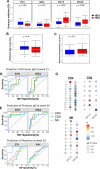
While preventing vertical HIV transmission has been very successful, the increasing number of HIV-exposed uninfected infants (iHEU) experience an elevated risk to infections compared to HIV-unexposed and uninfected infants (iHUU). Immune developmental differences between iHEU and iHUU remains poorly understood and here we present a longitudinal multimodal analysis of infant immune ontogeny that highlights the impact of HIV/ARV exposure. Using mass cytometry, we show alterations and differences in the emergence of NK cell populations and T cell memory differentiation between iHEU and iHUU. Specific NK cells observed at birth were also predictive of acellular pertussis and rotavirus vaccine-induced IgG and IgA responses, respectively, at 3 and 9 months of life. T cell receptor Vβ clonotypic diversity was significantly and persistently lower in iHEU preceding the expansion of T cell memory. Our findings show that HIV/ARV exposure disrupts innate and adaptive immunity from birth which may underlie relative vulnerability to infections.
Keywords: HIV exposure; NK cels and antibody responses; T cell receptor; infant immunity.
Conflict of interest statement
Declaration of Interest All authors declare no conflict of interest of any of the material included in this manuscript.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
