This is a preprint.
Infection-induced epilepsy is caused by increased expression of chondroitin sulfate proteoglycans in hippocampus and amygdala
- PMID: 37292901
- PMCID: PMC10245664
- DOI: 10.1101/2023.05.16.541066
Infection-induced epilepsy is caused by increased expression of chondroitin sulfate proteoglycans in hippocampus and amygdala
Update in
-
Increased expression of chondroitin sulfate proteoglycans in dentate gyrus and amygdala causes postinfectious seizures.Brain. 2024 May 3;147(5):1856-1870. doi: 10.1093/brain/awad430. Brain. 2024. PMID: 38146224 Free PMC article.
Abstract
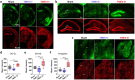
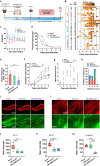
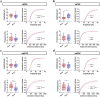
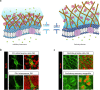
Alterations in the extracellular matrix (ECM) are common in epilepsy, yet whether they are cause or consequence of disease is unknow. Using Theiler's virus infection model of acquired epilepsy we find de novo expression of chondroitin sulfate proteoglycans (CSPGs), a major ECM component, in dentate gyrus (DG) and amygdala exclusively in mice with seizures. Preventing synthesis of CSPGs specifically in DG and amygdala by deletion of major CSPG aggrecan reduced seizure burden. Patch-clamp recordings from dentate granule cells (DGCs) revealed enhanced intrinsic and synaptic excitability in seizing mice that was normalized by aggrecan deletion. In situ experiments suggest that DGCs hyperexcitability results from negatively charged CSPGs increasing stationary cations (K+, Ca2+) on the membrane thereby depolarizing neurons, increasing their intrinsic and synaptic excitability. We show similar changes in CSPGs in pilocarpine-induced epilepsy suggesting enhanced CSPGs in the DG and amygdala may be a common ictogenic factor and novel therapeutic potential.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
