This is a preprint.
Neuropeptide Y signaling regulates recurrent excitation in the auditory midbrain
- PMID: 37292904
- PMCID: PMC10245754
- DOI: 10.1101/2023.05.16.540954
Neuropeptide Y signaling regulates recurrent excitation in the auditory midbrain
Update in
-
Neuropeptide Y Signaling Regulates Recurrent Excitation in the Auditory Midbrain.J Neurosci. 2023 Nov 8;43(45):7626-7641. doi: 10.1523/JNEUROSCI.0900-23.2023. Epub 2023 Sep 13. J Neurosci. 2023. PMID: 37704372 Free PMC article.
Abstract
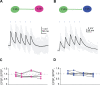
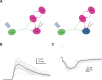
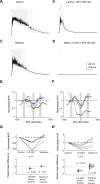
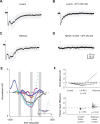
Neuropeptides play key roles in shaping the organization and function of neuronal circuits. In the inferior colliculus (IC), which is located in the auditory midbrain, Neuropeptide Y (NPY) is expressed by a large class of GABAergic neurons that project locally as well as outside the IC. The IC integrates information from numerous auditory nuclei making the IC an important hub for sound processing. Most neurons in the IC have local axon collaterals, however the organization and function of local circuits in the IC remains largely unknown. We previously found that neurons in the IC can express the NPY Y1 receptor (Y 1 R + ) and application of the Y 1 R agonist, [Leu 31 , Pro 34 ]-NPY (LP-NPY), decreases the excitability of Y 1 R + neurons. To investigate how Y 1 R + neurons and NPY signaling contribute to local IC networks, we used optogenetics to activate Y 1 R + neurons while recording from other neurons in the ipsilateral IC. Here, we show that 78.4% of glutamatergic neurons in the IC express the Y1 receptor, providing extensive opportunities for NPY signaling to regulate excitation in local IC circuits. Additionally, Y 1 R + neuron synapses exhibit modest short-term synaptic plasticity, suggesting that local excitatory circuits maintain their influence over computations during sustained stimuli. We further found that application of LP-NPY decreases recurrent excitation in the IC, suggesting that NPY signaling strongly regulates local circuit function in the auditory midbrain. Together, our data show that excitatory neurons are highly interconnected in the local IC and their influence over local circuits is tightly regulated by NPY signaling.
Conflict of interest statement
Conflict of Interest Statement: The authors declare no competing financial interests.
Figures
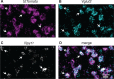
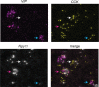
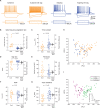
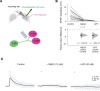

Similar articles
-
Neuropeptide Y Signaling Regulates Recurrent Excitation in the Auditory Midbrain.J Neurosci. 2023 Nov 8;43(45):7626-7641. doi: 10.1523/JNEUROSCI.0900-23.2023. Epub 2023 Sep 13. J Neurosci. 2023. PMID: 37704372 Free PMC article.
-
Neuropeptide Y Expression Defines a Novel Class of GABAergic Projection Neuron in the Inferior Colliculus.J Neurosci. 2020 Jun 10;40(24):4685-4699. doi: 10.1523/JNEUROSCI.0420-20.2020. Epub 2020 May 6. J Neurosci. 2020. PMID: 32376782 Free PMC article.
-
Lineage-tracing reveals an expanded population of NPY neurons in the inferior colliculus.bioRxiv [Preprint]. 2024 Mar 30:2024.03.27.587042. doi: 10.1101/2024.03.27.587042. bioRxiv. 2024. Update in: J Neurophysiol. 2024 Aug 1;132(2):573-588. doi: 10.1152/jn.00131.2024. PMID: 38585909 Free PMC article. Updated. Preprint.
-
Identifying neuron types and circuit mechanisms in the auditory midbrain.Hear Res. 2024 Feb;442:108938. doi: 10.1016/j.heares.2023.108938. Epub 2023 Dec 20. Hear Res. 2024. PMID: 38141518 Free PMC article. Review.
-
Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system.Brain Res Brain Res Rev. 1998 May;26(2-3):154-66. doi: 10.1016/s0165-0173(97)00052-0. Brain Res Brain Res Rev. 1998. PMID: 9651513 Review.
References
-
- Adams JC (1979) Ascending projections to the inferior colliculus. J Comp Neurol 183:519–538. - PubMed
-
- Bates, Mächler M, Bolker B, Waker S (2015) Fitting Linear Mixed-Effects Models Using lme4. J Stat Soft 67:1–48.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
