This is a preprint.
Distinct T cell functional profiles in SARS-CoV-2 seropositive and seronegative children associated with endemic human coronavirus cross-reactivity
- PMID: 37292954
- PMCID: PMC10246143
- DOI: 10.1101/2023.05.16.23290059
Distinct T cell functional profiles in SARS-CoV-2 seropositive and seronegative children associated with endemic human coronavirus cross-reactivity
Update in
-
Distinct T cell polyfunctional profile in SARS-CoV-2 seronegative children associated with endemic human coronavirus cross-reactivity.iScience. 2023 Dec 14;27(1):108728. doi: 10.1016/j.isci.2023.108728. eCollection 2024 Jan 19. iScience. 2023. PMID: 38235336 Free PMC article.
Abstract
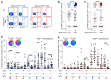
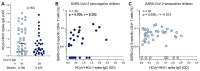
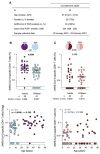
SARS-CoV-2 infection in children typically results in asymptomatic or mild disease. There is a paucity of studies on antiviral immunity in African children. We investigated SARS-CoV-2-specific T cell responses in 71 unvaccinated asymptomatic South African children who were seropositive or seronegative for SARS-CoV-2. SARS-CoV-2-specific CD4+ T cell responses were detectable in 83% of seropositive and 60% of seronegative children. Although the magnitude of the CD4+ T cell response did not differ significantly between the two groups, their functional profiles were distinct, with SARS-CoV-2 seropositive children exhibiting a higher proportion of polyfunctional T cells compared to their seronegative counterparts. The frequency of SARS-CoV-2-specific CD4+ T cells in seronegative children was associated with the endemic human coronavirus (HCoV) HKU1 IgG response. Overall, the presence of SARS-CoV-2-responding T cells in seronegative children may result from cross-reactivity to endemic coronaviruses and could contribute to the relative protection from disease observed in SARS-CoV-2-infected children.
Keywords: Children; IgG responses; Polyfunctional profile; SARS-CoV-2; T cell response; endemic HCoV.
Conflict of interest statement
DECLARATION OF INTERESTS The authors have no competing interests.
Figures

References
-
- UNICEF. COVID-19 confirmed cases and deaths, age and sex-disaggregated data. Published April 2023. https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-da.... Accessed April 27, 2023.
-
- Kim L, Whitaker M, Kambhampati A, et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 — COVID-NET, 14 States, March 1–July 25, 2020. 2020;69(32):1081–1088. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/purpose- - PMC - PubMed
-
- COVID-NET. COVID-19-Associated Hospitalization Surveillance Network, Centers for Disease Control and Prevention. Published April 2023. https://gis.cdc.gov/grasp/covidnet/covid19_5.html. Accessed April 27, 2023
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
