This is a preprint.
Endogenous pathology in tauopathy mice progresses via brain networks
- PMID: 37293074
- PMCID: PMC10245958
- DOI: 10.1101/2023.05.23.541792
Endogenous pathology in tauopathy mice progresses via brain networks
Update in
-
Spontaneous pathology in PS19 tauopathy mice progresses via brain networks.Neurobiol Dis. 2025 Oct 15;215:107072. doi: 10.1016/j.nbd.2025.107072. Epub 2025 Aug 29. Neurobiol Dis. 2025. PMID: 40886860
Abstract
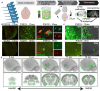
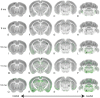
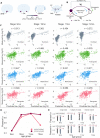
Neurodegenerative tauopathies are hypothesized to propagate via brain networks. This is uncertain because we have lacked precise network resolution of pathology. We therefore developed whole-brain staining methods with anti-p-tau nanobodies and imaged in 3D PS19 tauopathy mice, which have pan-neuronal expression of full-length human tau containing the P301S mutation. We analyzed patterns of p-tau deposition across established brain networks at multiple ages, testing the relationship between structural connectivity and patterns of progressive pathology. We identified core regions with early tau deposition, and used network propagation modeling to determine the link between tau pathology and connectivity strength. We discovered a bias towards retrograde network-based propagation of tau. This novel approach establishes a fundamental role for brain networks in tau propagation, with implications for human disease.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures

References
-
- Lee V. M., Goedert M., Trojanowski J. Q., Neurodegenerative tauopathies. Annu Rev Neurosci 24, 1121–1159 (2001). - PubMed
