This is a preprint.
Divergent host innate immune response to the smooth-to-rough M. abscessus adaptation to chronic infection
- PMID: 37293112
- PMCID: PMC10245581
- DOI: 10.1101/2023.05.15.540822
Divergent host innate immune response to the smooth-to-rough M. abscessus adaptation to chronic infection
Update in
-
Divergent host humoral innate immune response to the smooth-to-rough adaptation of Mycobacterium abscessus in chronic infection.Front Cell Infect Microbiol. 2025 Mar 18;15:1445660. doi: 10.3389/fcimb.2025.1445660. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40171164 Free PMC article.
Abstract
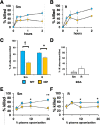
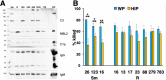
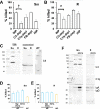
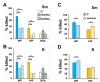
Mycobacterium abscessus is a nontuberculous mycobacterium emerging as a significant pathogen for individuals with chronic lung disease, including cystic fibrosis and chronic obstructive pulmonary disease. Current therapeutics have poor efficacy. New strategies of bacterial control based on host defenses are appealing, but anti-mycobacterial immune mechanisms are poorly understood and are complicated by the appearance of smooth and rough morphotypes with distinct host responses. We explored the role of the complement system in the clearance of M. abscessus morphotypes by neutrophils, an abundant cell in these infections. M. abscessus opsonized with plasma from healthy individuals promoted greater killing by neutrophils compared to opsonization in heat-inactivated plasma. Rough clinical isolates were more resistant to complement but were still efficiently killed. Complement C3 associated strongly with the smooth morphotype while mannose-binding lectin 2 was associated with the rough morphotype. M. abscessus killing was dependent on C3, but not on C1q or Factor B; furthermore, competition of mannose-binding lectin 2 binding with mannan or N-acetyl-glucosamine during opsonization did not inhibit killing. These data suggest that M. abscessus does not canonically activate complement through the classical, alternative, or lectin pathways. Complement-mediated killing was dependent on IgG and IgM for smooth and on IgG for rough M. abscessus. Both morphotypes were recognized by Complement Receptor 3 (CD11b), but not CR1 (CD35), and in a carbohydrate- and calcium-dependent manner. These data suggest the smooth-to-rough adaptation changes complement recognition of M. abscessus and that complement is an important factor for M. abscessus infection.
Figures




References
-
- Nick JA, Daley CL, Lenhart-Pendergrass PM, Davidson RM. 2021. Nontuberculous mycobacteria in cystic fibrosis. Curr Opin Pulm Med 27:586–592. - PubMed
-
- Nick JA, Dedrick RM, Gray AL, Vladar EK, Smith BE, Freeman KG, Malcolm KC, Epperson LE, Hasan NA, Hendrix J, Callahan K, Walton K, Vestal B, Wheeler E, Rysavy NM, Poch K, Caceres S, Lovell VK, Hisert KB, de Moura VC, Chatterjee D, De P, Weakly N, Martiniano SL, Lynch DA, Daley CL, Strong M, Jia F, Hatfull GF, Davidson RM. 2022. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 185:1860–1874 e1812. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
