Cell-specific transcriptome changes in the hypothalamic arcuate nucleus in a mouse deoxycorticosterone acetate-salt model of hypertension
- PMID: 37293629
- PMCID: PMC10244568
- DOI: 10.3389/fncel.2023.1207350
Cell-specific transcriptome changes in the hypothalamic arcuate nucleus in a mouse deoxycorticosterone acetate-salt model of hypertension
Abstract
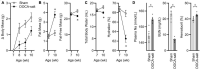
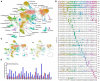
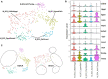
A common preclinical model of hypertension characterized by low circulating renin is the "deoxycorticosterone acetate (DOCA)-salt" model, which influences blood pressure and metabolism through mechanisms involving the angiotensin II type 1 receptor (AT1R) in the brain. More specifically, AT1R within Agouti-related peptide (AgRP) neurons of the arcuate nucleus of the hypothalamus (ARC) has been implicated in selected effects of DOCA-salt. In addition, microglia have been implicated in the cerebrovascular effects of DOCA-salt and angiotensin II. To characterize DOCA-salt effects upon the transcriptomes of individual cell types within the ARC, we used single-nucleus RNA sequencing (snRNAseq) to examine this region from male C57BL/6J mice that underwent sham or DOCA-salt treatment. Thirty-two unique primary cell type clusters were identified. Sub-clustering of neuropeptide-related clusters resulted in identification of three distinct AgRP subclusters. DOCA-salt treatment caused subtype-specific changes in gene expression patterns associated with AT1R and G protein signaling, neurotransmitter uptake, synapse functions, and hormone secretion. In addition, two primary cell type clusters were identified as resting versus activated microglia, and multiple distinct subtypes of activated microglia were suggested by sub-cluster analysis. While DOCA-salt had no overall effect on total microglial density within the ARC, DOCA-salt appeared to cause a redistribution of the relative abundance of activated microglia subtypes. These data provide novel insights into cell-specific molecular changes occurring within the ARC during DOCA-salt treatment, and prompt increased investigation of the physiological and pathophysiological significance of distinct subtypes of neuronal and glial cell types.
Keywords: AgRP neurons; DOCA-salt; arcuate nucleus; microglia; mouse; snRNAseq.
Copyright © 2023 Wagner, Deng, Claflin, Ritter, Cui, Nakagawa, Sigmund, Morselli, Grobe and Kwitek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Buffolo F., Monticone S., Pecori A., Pieroni J., Losano I., Cavaglià G., et al. (2020). The spectrum of low-renin hypertension. Best Pract. Res. Clin. Endocrinol. Metab. 34:101399. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

