Starburst amacrine cells form gap junctions in the early postnatal stage of the mouse retina
- PMID: 37293630
- PMCID: PMC10244514
- DOI: 10.3389/fncel.2023.1173579
Starburst amacrine cells form gap junctions in the early postnatal stage of the mouse retina
Abstract
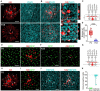
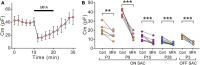
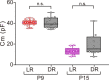
Although gap junctional coupling in the developing retina is important for the maturation of neuronal networks, its role in the development of individual neurons remains unclear. Therefore, we herein investigated whether gap junctional coupling by starburst amacrine cells (SACs), a key neuron for the formation of direction selectivity, occurs during the developmental stage in the mouse retina. Neurobiotin-injected SACs coupled with many neighboring cells before eye-opening. The majority of tracer-coupled cells were retinal ganglion cells, and tracer coupling was not detected between SACs. The number of tracer-coupled cells significantly decreased after eye-opening and mostly disappeared by postnatal day 28 (P28). Membrane capacitance (Cm), an indicator of the formation of electrical coupling with gap junctions, was larger in SACs before than after eye-opening. The application of meclofenamic acid, a gap junction blocker, reduced the Cm of SACs. Gap junctional coupling by SACs was regulated by dopamine D1 receptors before eye-opening. In contrast, the reduction in gap junctional coupling after eye-opening was not affected by visual experience. At the mRNA level, 4 subtypes of connexins (23, 36, 43, and 45) were detected in SACs before eye-opening. Connexin 43 expression levels significantly decreased after eye-opening. These results indicate that gap junctional coupling by SACs occurs during the developmental period and suggest that the elimination of gap junctions proceeds with the innate system.
Keywords: connexin (Cx); development; dopamine D1 receptor (D1R); gap junction; retina; retinal ganglion cell (RGC); starburst amacrine cells (SACs); visual experience.
Copyright © 2023 Maruyama, Ishii and Kaneda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina.J Comp Neurol. 1997 Jul 14;383(4):512-28. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. J Comp Neurol. 1997. PMID: 9208996
-
Cell firing between ON alpha retinal ganglion cells and coupled amacrine cells in the mouse retina.Am J Physiol Cell Physiol. 2024 Sep 1;327(3):C716-C727. doi: 10.1152/ajpcell.00238.2024. Epub 2024 Jul 16. Am J Physiol Cell Physiol. 2024. PMID: 39010839
-
Amacrine Cells Forming Gap Junctions With Intrinsically Photosensitive Retinal Ganglion Cells: ipRGC Types, Neuromodulator Contents, and Connexin Isoform.Invest Ophthalmol Vis Sci. 2021 Jan 4;62(1):10. doi: 10.1167/iovs.62.1.10. Invest Ophthalmol Vis Sci. 2021. PMID: 33410914 Free PMC article.
-
Gap junctional coupling in the vertebrate retina: variations on one theme?Prog Retin Eye Res. 2013 May;34:1-18. doi: 10.1016/j.preteyeres.2012.12.002. Epub 2013 Jan 8. Prog Retin Eye Res. 2013. PMID: 23313713 Review.
-
Temporal expression of neuronal connexins during hippocampal ontogeny.Brain Res Brain Res Rev. 2000 Apr;32(1):57-71. doi: 10.1016/s0165-0173(99)00096-x. Brain Res Brain Res Rev. 2000. PMID: 10751657 Review.
Cited by
-
Gene-environmental regulation of the postnatal post-mitotic neuronal maturation.Trends Genet. 2024 Jun;40(6):480-494. doi: 10.1016/j.tig.2024.03.006. Epub 2024 Apr 23. Trends Genet. 2024. PMID: 38658255 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

