Pillar-layer Zn-triazolate-dicarboxylate frameworks with a customized pore structure for efficient ethylene purification from ethylene/ethane/acetylene ternary mixtures
- PMID: 37293648
- PMCID: PMC10246669
- DOI: 10.1039/d3sc01134h
Pillar-layer Zn-triazolate-dicarboxylate frameworks with a customized pore structure for efficient ethylene purification from ethylene/ethane/acetylene ternary mixtures
Abstract
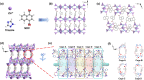
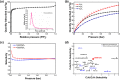
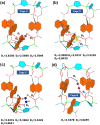
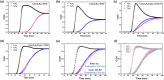
The selective adsorption of C2H6 and C2H2 over C2H4 from C2H2/C2H4/C2H6 ternary mixtures for one-step C2H4 purification represents a crucial yet challenging task in industry. The pore structure of the adsorbents must be finely tailored to meet the demanding requirements for the separation considering the very similar physicochemical properties of the three gases. Herein, we report a Zn-triazolate-dicarboxylate framework, HIAM-210, featuring a novel topology which possesses one-dimensional channels decorated with adjacent uncoordinated carboxylate-O atoms. The suitable pore size and customized pore environment enable the compound to selectively capture C2H6 and C2H2 with high C2H2/C2H4 and C2H6/C2H4 selectivities of both 2.0. Breakthrough experiments show that polymer-grade C2H4 can be directly harvested from C2H2/C2H4/C2H6 (34/33/33 and 1/90/9) ternary mixtures. The underlying mechanism of the preferential adsorption was uncovered by grand canonical Monte Carlo simulations and DFT calculations.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Immobilization of Lewis Basic Sites into a Stable Ethane-Selective MOF Enabling One-Step Separation of Ethylene from a Ternary Mixture.J Am Chem Soc. 2022 Feb 16;144(6):2614-2623. doi: 10.1021/jacs.1c10973. Epub 2022 Feb 2. J Am Chem Soc. 2022. PMID: 35109657
-
Ultramicroporous Metal-Organic Framework with Inert Pore Surfaces for Inversed Separation of Ethylene from C2 Hydrocarbons Mixtures.ACS Appl Mater Interfaces. 2023 May 17;15(19):23538-23545. doi: 10.1021/acsami.3c04225. Epub 2023 May 7. ACS Appl Mater Interfaces. 2023. PMID: 37150971
-
Tailoring a robust Al-MOF for trapping C2H6 and C2H2 towards efficient C2H4 purification from quaternary mixtures.Chem Sci. 2022 May 20;13(24):7172-7180. doi: 10.1039/d2sc01180h. eCollection 2022 Jun 22. Chem Sci. 2022. PMID: 35799813 Free PMC article.
-
One-step ethylene production from a four-component gas mixture by a single physisorbent.Nat Commun. 2021 Nov 11;12(1):6507. doi: 10.1038/s41467-021-26473-8. Nat Commun. 2021. PMID: 34764243 Free PMC article.
-
Tailoring Metal-Organic Frameworks for One-Step Separation of Alkane/Alkene/Alkyne Mixtures.Chem Asian J. 2025 Apr 3;20(7):e202401529. doi: 10.1002/asia.202401529. Epub 2025 Feb 1. Chem Asian J. 2025. PMID: 39800887 Review.
Cited by
-
Excitation generated preferential binding sites for ethane on porous carbon-copper porphyrin sorbents: ethane/ethylene adsorptive separation improved by light.Chem Sci. 2024 Apr 16;15(19):7285-7292. doi: 10.1039/d4sc00898g. eCollection 2024 May 15. Chem Sci. 2024. PMID: 38756801 Free PMC article.
References
-
- Ding Q. Zhang Z. Liu Y. Chai K. Krishna R. Zhang S. One-Step Ethylene Purification from Ternary Mixtures in a Metal-Organic Framework with Customized Pore Chemistry and Shape. Angew. Chem., Int. Ed. Engl. 2022;61:e202208134. - PubMed
-
- Yoshimura Y. Kijima N. Hayakawa T. Murata K. Suzuki K. Mizukami F. Matano K. Konishi T. Oikawa T. Saito M. Catalytic cracking of naphtha to light olefins. Catal. Surv. Jpn. 2001;4:157–167. doi: 10.1023/A:1011463606189. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

