Phenotype switching of the mutation rate facilitates adaptive evolution
- PMID: 37293818
- PMCID: PMC10471227
- DOI: 10.1093/genetics/iyad111
Phenotype switching of the mutation rate facilitates adaptive evolution
Abstract
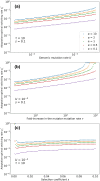
The mutation rate plays an important role in adaptive evolution. It can be modified by mutator and anti-mutator alleles. Recent empirical evidence hints that the mutation rate may vary among genetically identical individuals: evidence from bacteria suggests that the mutation rate can be affected by expression noise of a DNA repair protein and potentially also by translation errors in various proteins. Importantly, this non-genetic variation may be heritable via a transgenerational epigenetic mode of inheritance, giving rise to a mutator phenotype that is independent from mutator alleles. Here, we investigate mathematically how the rate of adaptive evolution is affected by the rate of mutation rate phenotype switching. We model an asexual population with two mutation rate phenotypes, non-mutator and mutator. An offspring may switch from its parental phenotype to the other phenotype. We find that switching rates that correspond to so-far empirically described non-genetic systems of inheritance of the mutation rate lead to higher rates of adaptation on both artificial and natural fitness landscapes. These switching rates can maintain within the same individuals both a mutator phenotype and intermediary mutations, a combination that facilitates adaptation. Moreover, non-genetic inheritance increases the proportion of mutators in the population, which in turn increases the probability of hitchhiking of the mutator phenotype with adaptive mutations. This in turns facilitates the acquisition of additional adaptive mutations. Our results rationalize recently observed noise in the expression of proteins that affect the mutation rate and suggest that non-genetic inheritance of this phenotype may facilitate evolutionary adaptive processes.
Keywords: epigenetics; evolutionary adaptive process; mutation rate; mutation rate phenotype switching; non-genetic inheritance; non-genetic variation.
© The Author(s) 2023. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest: The author(s) declare no conflict of interest.
Figures




Similar articles
-
Mutators, population size, adaptive landscape and the adaptation of asexual populations of bacteria.Genetics. 1999 Jun;152(2):485-93. doi: 10.1093/genetics/152.2.485. Genetics. 1999. PMID: 10353893 Free PMC article.
-
Genetic adaptation of Pseudomonas aeruginosa during chronic lung infection of patients with cystic fibrosis: strong and weak mutators with heterogeneous genetic backgrounds emerge in mucA and/or lasR mutants.Microbiology (Reading). 2010 Apr;156(Pt 4):1108-1119. doi: 10.1099/mic.0.033993-0. Epub 2009 Dec 17. Microbiology (Reading). 2010. PMID: 20019078
-
Evolution of Mutation Rates in Rapidly Adapting Asexual Populations.Genetics. 2016 Nov;204(3):1249-1266. doi: 10.1534/genetics.116.193565. Epub 2016 Sep 19. Genetics. 2016. PMID: 27646140 Free PMC article.
-
Transient and heritable mutators in adaptive evolution in the lab and in nature.Genetics. 1998 Apr;148(4):1559-66. doi: 10.1093/genetics/148.4.1559. Genetics. 1998. PMID: 9560375 Free PMC article. Review.
-
Signification and Application of Mutator and Antimutator Phenotype-Induced Genetic Variations in Evolutionary Adaptation and Cancer Therapeutics.J Microbiol. 2023 Dec;61(12):1013-1024. doi: 10.1007/s12275-023-00091-z. Epub 2023 Dec 15. J Microbiol. 2023. PMID: 38100001 Review.
Cited by
-
Biocides as drivers of antibiotic resistance: A critical review of environmental implications and public health risks.Environ Sci Ecotechnol. 2025 Mar 24;25:100557. doi: 10.1016/j.ese.2025.100557. eCollection 2025 May. Environ Sci Ecotechnol. 2025. PMID: 40230384 Free PMC article. Review.
-
The causal arrows from genotype, environment, and management to plant phenotype are double headed.J Exp Bot. 2025 Feb 25;76(4):917-930. doi: 10.1093/jxb/erae455. J Exp Bot. 2025. PMID: 39545971 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

