Dietary nitrate preserves mitochondrial bioenergetics and mitochondrial protein synthesis rates during short-term immobilization in mice
- PMID: 37293995
- PMCID: PMC12306412
- DOI: 10.1113/JP284701
Dietary nitrate preserves mitochondrial bioenergetics and mitochondrial protein synthesis rates during short-term immobilization in mice
Abstract
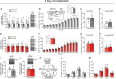
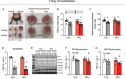
Skeletal muscle disuse reduces muscle protein synthesis rates and induces atrophy, events associated with decreased mitochondrial respiration and increased reactive oxygen species. Given that dietary nitrate can improve mitochondrial bioenergetics, we examined whether nitrate supplementation attenuates disuse-induced impairments in mitochondrial function and muscle protein synthesis rates. Female C57Bl/6N mice were subjected to single-limb casting (3 or 7 days) and consumed drinking water with or without 1 mM sodium nitrate. Compared with the contralateral control limb, 3 days of immobilization lowered myofibrillar fractional synthesis rates (FSR, P < 0.0001), resulting in muscle atrophy. Although FSR and mitophagy-related proteins were higher in subsarcolemmal (SS) compared with intermyofibrillar (IMF) mitochondria, immobilization for 3 days decreased FSR in both SS (P = 0.009) and IMF (P = 0.031) mitochondria. Additionally, 3 days of immobilization reduced maximal mitochondrial respiration, decreased mitochondrial protein content, and increased maximal mitochondrial reactive oxygen species emission, without altering mitophagy-related proteins in muscle homogenate or isolated mitochondria (SS and IMF). Although nitrate consumption did not attenuate the decline in muscle mass or myofibrillar FSR, intriguingly, nitrate completely prevented immobilization-induced reductions in SS and IMF mitochondrial FSR. In addition, nitrate prevented alterations in mitochondrial content and bioenergetics after both 3 and 7 days of immobilization. However, in contrast to 3 days of immobilization, nitrate did not prevent the decline in SS and IMF mitochondrial FSR after 7 days of immobilization. Therefore, although nitrate supplementation was not sufficient to prevent muscle atrophy, nitrate may represent a promising therapeutic strategy to maintain mitochondrial bioenergetics and transiently preserve mitochondrial protein synthesis rates during short-term muscle disuse. KEY POINTS: Alterations in mitochondrial bioenergetics (decreased respiration and increased reactive oxygen species) are thought to contribute to muscle atrophy and reduced protein synthesis rates during muscle disuse. Given that dietary nitrate can improve mitochondrial bioenergetics, we examined whether nitrate supplementation could attenuate immobilization-induced skeletal muscle impairments in female mice. Dietary nitrate prevented short-term (3 day) immobilization-induced declines in mitochondrial protein synthesis rates, reductions in markers of mitochondrial content, and alterations in mitochondrial bioenergetics. Despite these benefits and the preservation of mitochondrial content and bioenergetics during more prolonged (7 day) immobilization, nitrate consumption did not preserve skeletal muscle mass or myofibrillar protein synthesis rates. Overall, although dietary nitrate did not prevent atrophy, nitrate supplementation represents a promising nutritional approach to preserve mitochondrial function during muscle disuse.
Keywords: immobilization; intermyofibrillar mitochondria; mitochondrial reactive oxygen species; mitochondrial respiration; nitrate; protein synthesis; subsarcolemmal mitochondria.
© 2023 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
A multimodal exercise countermeasure prevents the negative impact of head-down tilt bed rest on muscle volume and mitochondrial health in older adults.J Physiol. 2025 Jul;603(13):3813-3836. doi: 10.1113/JP285897. Epub 2024 Jun 15. J Physiol. 2025. PMID: 38878232 Free PMC article.
-
Acute mitochondrial reactive oxygen species emissions drive mitochondrial dysfunction after traumatic muscle injury in male mice.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C235-C250. doi: 10.1152/ajpcell.00407.2025. Epub 2025 Jun 4. Am J Physiol Cell Physiol. 2025. PMID: 40465482 Free PMC article.
-
Perspectives on the interpretation of mitochondrial responses during skeletal muscle disuse-induced atrophy.J Physiol. 2025 Jul;603(13):3679-3699. doi: 10.1113/JP284160. Epub 2025 May 5. J Physiol. 2025. PMID: 40321007 Free PMC article. Review.
-
A single bout of prior resistance exercise attenuates muscle atrophy and declines in myofibrillar protein synthesis during bed-rest in older men.J Physiol. 2025 Jan;603(1):87-105. doi: 10.1113/JP285130. Epub 2023 Oct 19. J Physiol. 2025. PMID: 37856286 Free PMC article.
-
Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: a systematic review.Sports Med. 2014 May;44(5):655-70. doi: 10.1007/s40279-013-0137-7. Sports Med. 2014. PMID: 24435468
Cited by
-
Salivary-Gland-Mediated Nitrate Recirculation as a Modulator for Cardiovascular Diseases.Biomolecules. 2025 Mar 19;15(3):439. doi: 10.3390/biom15030439. Biomolecules. 2025. PMID: 40149975 Free PMC article. Review.
-
Skeletal muscle immobilisation-induced atrophy: mechanistic insights from human studies.Clin Sci (Lond). 2024 Jun 19;138(12):741-756. doi: 10.1042/CS20231198. Clin Sci (Lond). 2024. PMID: 38895777 Free PMC article. Review.
-
Beeting atrophy: dietary nitrate to protect the powerhouse of the cell?J Physiol. 2025 Jul;603(13):3861-3862. doi: 10.1113/JP285115. Epub 2023 Jul 31. J Physiol. 2025. PMID: 37519113 Free PMC article. No abstract available.
-
The role of mitochondrial dynamics and mitophagy in skeletal muscle atrophy: from molecular mechanisms to therapeutic insights.Cell Mol Biol Lett. 2024 Apr 23;29(1):59. doi: 10.1186/s11658-024-00572-y. Cell Mol Biol Lett. 2024. PMID: 38654156 Free PMC article. Review.
-
Type 2 diabetes-related sarcopenia: role of nitric oxide.Nutr Metab (Lond). 2024 Dec 18;21(1):107. doi: 10.1186/s12986-024-00883-z. Nutr Metab (Lond). 2024. PMID: 39695784 Free PMC article. Review.
References
-
- Adhihetty, P. J. , Ljubicic, V. , Menzies, K. J. , & Hood, D. A. (2005). Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. American Journal of Physiology. Cell Physiology, 289(4), C994–C1001. - PubMed
-
- Bizeau, M. E. , Willis, W. T. , & Hazel, J. R. (1998). Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. Journal of Applied Physiology, 85(4), 1279–1284. - PubMed
-
- Brook, M. S. , Stokes, T. , Gorissen, S. H. M. , Bass, J. J. , McGlory, C. , Cegielski, J. , Wilkinson, D. J. , Phillips, B. E. , Smith, K. , Phillips, S. M. , & Atherton, P. J. (2022). Declines in muscle protein synthesis account for short‐term muscle disuse atrophy in humans in the absence of increased muscle protein breakdown. Journal of Cachexia, Sarcopenia and Muscle, 13(4), 2005–2016. - PMC - PubMed
-
- Brunetta, H. S. , Petrick, H. L. , Momken, I. , Handy, R. M. , Pignanelli, C. , Nunes, E. A. , Piquereau, J. , Mericskay, M. , & Holloway, G. P. (2022). Nitrate consumption preserves HFD‐induced skeletal muscle mitochondrial ADP sensitivity and lysine acetylation: A potential role for SIRT1. Redox Biology, 52, 102307. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
