Alpha-1-antitrypsin antagonizes COVID-19: a review of the epidemiology, molecular mechanisms, and clinical evidence
- PMID: 37294003
- PMCID: PMC10317171
- DOI: 10.1042/BST20230078
Alpha-1-antitrypsin antagonizes COVID-19: a review of the epidemiology, molecular mechanisms, and clinical evidence
Abstract
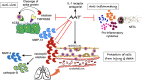
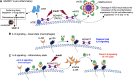
Alpha-1-antitrypsin (AAT), a serine protease inhibitor (serpin), is increasingly recognized to inhibit SARS-CoV-2 infection and counter many of the pathogenic mechanisms of COVID-19. Herein, we reviewed the epidemiologic evidence, the molecular mechanisms, and the clinical evidence that support this paradigm. As background to our discussion, we first examined the basic mechanism of SARS-CoV-2 infection and contend that despite the availability of vaccines and anti-viral agents, COVID-19 remains problematic due to viral evolution. We next underscored that measures to prevent severe COVID-19 currently exists but teeters on a balance and that current treatment for severe COVID-19 remains grossly suboptimal. We then reviewed the epidemiologic and clinical evidence that AAT deficiency increases risk of COVID-19 infection and of more severe disease, and the experimental evidence that AAT inhibits cell surface transmembrane protease 2 (TMPRSS2) - a host serine protease required for SARS-CoV-2 entry into cells - and that this inhibition may be augmented by heparin. We also elaborated on the panoply of other activities of AAT (and heparin) that could mitigate severity of COVID-19. Finally, we evaluated the available clinical evidence for AAT treatment of COVID-19.
Keywords: COVID-19; SARS-CoV-2; alpha-1-antitrypsin; heparin; serine protease; serpin.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Similar articles
-
Hypothesis: Alpha-1-antitrypsin is a promising treatment option for COVID-19.Med Hypotheses. 2021 Jan;146:110394. doi: 10.1016/j.mehy.2020.110394. Epub 2020 Nov 12. Med Hypotheses. 2021. PMID: 33239231 Free PMC article. Review.
-
Enoxaparin augments alpha-1-antitrypsin inhibition of TMPRSS2, a promising drug combination against COVID-19.Sci Rep. 2022 Mar 25;12(1):5207. doi: 10.1038/s41598-022-09133-9. Sci Rep. 2022. PMID: 35338216 Free PMC article.
-
d-Galactose treatment increases ACE2, TMPRSS2, and FURIN and reduces SERPINA1 mRNA expression in A549 human lung epithelial cells.Drug Dev Res. 2022 May;83(3):622-627. doi: 10.1002/ddr.21891. Epub 2021 Oct 22. Drug Dev Res. 2022. PMID: 34677831
-
Serine Protease Inhibitors Restrict Host Susceptibility to SARS-CoV-2 Infections.mBio. 2022 Jun 28;13(3):e0089222. doi: 10.1128/mbio.00892-22. Epub 2022 May 9. mBio. 2022. PMID: 35532162 Free PMC article.
-
A Review of Alpha-1 Antitrypsin Binding Partners for Immune Regulation and Potential Therapeutic Application.Int J Mol Sci. 2022 Feb 23;23(5):2441. doi: 10.3390/ijms23052441. Int J Mol Sci. 2022. PMID: 35269582 Free PMC article. Review.
Cited by
-
Bipotential B-neutrophil progenitors are present in human and mouse bone marrow and emerge in the periphery upon stress hematopoiesis.mBio. 2024 Aug 14;15(8):e0159924. doi: 10.1128/mbio.01599-24. Epub 2024 Jul 16. mBio. 2024. PMID: 39012145 Free PMC article.
-
Functional mass spectrometry indicates anti-protease and complement activity increase with COVID-19 severity.Exp Biol Med (Maywood). 2025 Jan 29;250:10308. doi: 10.3389/ebm.2025.10308. eCollection 2025. Exp Biol Med (Maywood). 2025. PMID: 39949890 Free PMC article.
-
Research Trends in Proteomic Studies Using Serum from COVID-19 Patients: A Bibliometric Analysis.Curr Med Chem. 2025;32(12):2275-2290. doi: 10.2174/0109298673286915240329063441. Curr Med Chem. 2025. PMID: 38584539
-
The Inhibition of Serine Proteases by Serpins Is Augmented by Negatively Charged Heparin: A Concise Review of Some Clinically Relevant Interactions.Int J Mol Sci. 2024 Feb 2;25(3):1804. doi: 10.3390/ijms25031804. Int J Mol Sci. 2024. PMID: 38339082 Free PMC article. Review.
-
Analysis of alpha-1-antitrypsin (AAT)-regulated, glucocorticoid receptor-dependent genes in macrophages reveals a novel host defense function of AAT.Physiol Rep. 2024 Jul;12(14):e16124. doi: 10.14814/phy2.16124. Physiol Rep. 2024. PMID: 39016119 Free PMC article.
References
-
- (2022) Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingco...
-
- (2022) Centers for Disease Control and Prevention. Science brief: Evidence used to update the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19 https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingco...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

