Pan-cancer analysis identifies LPCATs family as a prognostic biomarker and validation of LPCAT4/WNT/β-catenin/c-JUN/ACSL3 in hepatocellular carcinoma
- PMID: 37294538
- PMCID: PMC10292872
- DOI: 10.18632/aging.204723
Pan-cancer analysis identifies LPCATs family as a prognostic biomarker and validation of LPCAT4/WNT/β-catenin/c-JUN/ACSL3 in hepatocellular carcinoma
Abstract
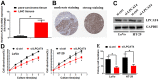
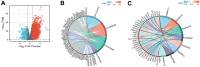
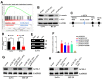
Lipid remodeling regulators are now being investigated as potential therapeutic targets for cancer therapy as a result of their involvement, which includes promoting cancer cells' adaptation to the restricted environment. Lysophosphatidylcholine acyltransferases (LPCATs, LPCAT1-4) are enzymes that regulate the remodeling of bio-membranes. The functions of these enzymes in cancer are largely unknown. In the current study, we found that genes belonging to the LPCAT family participated in tumor advancement and were strongly linked to dismal prognosis in many different malignancies. We constructed the LPCATs scores model and explored this model in pan-cancer. Malignant pathways in pan-cancer were positively related to LPCATs scores, and all pathways had strong links to the tumor microenvironment (TME). Multiple immune-associated features of the TME in pan-cancer were likewise associated with higher LPCATs scores. In addition, the LPCATs score functioned as a prognostic marker for immune checkpoint inhibitor (ICI) therapies in patients with cancer. LPCAT4 enhanced cell growth and cholesterol biosynthesis by up-regulating ACSL3 in hepatocellular carcinoma (HCC). WNT/β-catenin/c-JUN signaling pathway mediated LPCAT4's regulation on ACSL3. These findings demonstrated that genes in the LPCAT family might be used as cancer immunotherapy and prognosis-related biomarkers. Specifically, LPCAT4 could be a treatment target of HCC.
Keywords: LPCATs; bioinformatics; cholesterol biosynthesis; liver hepatocellular carcinoma.
Conflict of interest statement
Figures





Similar articles
-
Comprehensive Analysis of LPCATs Highlights the Prognostic and Immunological Values of LPCAT1/4 in Hepatocellular Carcinoma.Int J Gen Med. 2021 Nov 30;14:9117-9130. doi: 10.2147/IJGM.S344723. eCollection 2021. Int J Gen Med. 2021. PMID: 34876845 Free PMC article.
-
LPCAT1 acts as an independent prognostic biomarker correlated with immune infiltration in hepatocellular carcinoma.Eur J Med Res. 2022 Oct 28;27(1):216. doi: 10.1186/s40001-022-00854-1. Eur J Med Res. 2022. PMID: 36307879 Free PMC article.
-
Lysophosphatidylcholine acyltransferase 1 altered phospholipid composition and regulated hepatoma progression.J Hepatol. 2013 Aug;59(2):292-9. doi: 10.1016/j.jhep.2013.02.030. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567080
-
Targeting Wnt/β-catenin pathway in hepatocellular carcinoma treatment.World J Gastroenterol. 2016 Jan 14;22(2):823-32. doi: 10.3748/wjg.v22.i2.823. World J Gastroenterol. 2016. PMID: 26811628 Free PMC article. Review.
-
Phospholipid Remodeling in Physiology and Disease.Annu Rev Physiol. 2019 Feb 10;81:165-188. doi: 10.1146/annurev-physiol-020518-114444. Epub 2018 Oct 31. Annu Rev Physiol. 2019. PMID: 30379616 Free PMC article. Review.
Cited by
-
Reprogrammed immuno-metabolic environment of cancer: the driving force of ferroptosis resistance.Mol Cancer. 2025 Jun 3;24(1):161. doi: 10.1186/s12943-025-02337-3. Mol Cancer. 2025. PMID: 40462094 Free PMC article. Review.
-
Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases.Cancers (Basel). 2024 May 31;16(11):2115. doi: 10.3390/cancers16112115. Cancers (Basel). 2024. PMID: 38893234 Free PMC article. Review.
-
Investigating the causal relationship between the plasma lipidome and cholangiocarcinoma mediated by immune cells: a mediation Mendelian randomization study.Sci Rep. 2025 Feb 17;15(1):5807. doi: 10.1038/s41598-025-90140-x. Sci Rep. 2025. PMID: 39962308 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

