Effect of Aspirin vs Enoxaparin on 90-Day Mortality in Patients Undergoing Hip or Knee Arthroplasty: A Secondary Analysis of the CRISTAL Cluster Randomized Trial
- PMID: 37294566
- PMCID: PMC10257098
- DOI: 10.1001/jamanetworkopen.2023.17838
Effect of Aspirin vs Enoxaparin on 90-Day Mortality in Patients Undergoing Hip or Knee Arthroplasty: A Secondary Analysis of the CRISTAL Cluster Randomized Trial
Abstract
Importance: Ischemic heart disease remains the leading cause of mortality following hip and knee arthroplasty. Due to its antiplatelet and cardioprotective properties, aspirin has been proposed as an agent that could reduce mortality when used as venous thromboembolism (VTE) prophylaxis following these procedures.
Objective: To compare aspirin with enoxaparin in reducing 90-day mortality for patients undergoing hip or knee arthroplasty procedures.
Design, setting, and participants: This study was a planned secondary analysis of the CRISTAL cluster randomized, crossover, registry-nested trial performed across 31 participating hospitals in Australia between April 20, 2019, and December 18, 2020. The aim of the CRISTAL trial was to determine whether aspirin was noninferior to enoxaparin in preventing symptomatic VTE following hip or knee arthroplasty. The primary study restricted the analysis to patients undergoing total hip or knee arthroplasty for a diagnosis of osteoarthritis only. This study includes all adult patients (aged ≥18 years) undergoing any hip or knee arthroplasty procedure at participating sites during the course of the trial. Data were analyzed from June 1 to September 6, 2021.
Interventions: Hospitals were randomized to administer all patients oral aspirin (100 mg daily) or subcutaneous enoxaparin (40 mg daily) for 35 days after hip arthroplasty and 14 days after knee arthroplasty procedures.
Main outcomes and measures: The primary outcome was mortality within 90 days. The between-group difference in mortality was estimated using cluster summary methods.
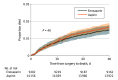
Results: A total of 23 458 patients from 31 hospitals were included, with 14 156 patients allocated to aspirin (median [IQR] age, 69 [62-77] years; 7984 [56.4%] female) and 9302 patients allocated to enoxaparin (median [IQR] age, 70 [62-77] years; 5277 [56.7%] female). The mortality rate within 90 days of surgery was 1.67% in the aspirin group and 1.53% in the enoxaparin group (estimated difference, 0.04%; 95% CI, -0.05%-0.42%). For the subgroup of 21 148 patients with a nonfracture diagnosis, the mortality rate was 0.49% in the aspirin group and 0.41% in the enoxaparin group (estimated difference, 0.05%; 95% CI, -0.67% to 0.76%).
Conclusions and relevance: In this secondary analysis of a cluster randomized trial comparing aspirin with enoxaparin following hip or knee arthroplasty, there was no significant between-group difference in mortality within 90 days when either drug was used for VTE prophylaxis.
Trial registration: http://anzctr.org.au Identifier: ACTRN12618001879257.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous