Rapid Kinetics of Pistol Ribozyme: Insights into Limits to RNA Catalysis
- PMID: 37294744
- PMCID: PMC10330772
- DOI: 10.1021/acs.biochem.3c00160
Rapid Kinetics of Pistol Ribozyme: Insights into Limits to RNA Catalysis
Abstract
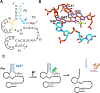
Pistol ribozyme (Psr) is a distinct class of small endonucleolytic ribozymes, which are important experimental systems for defining fundamental principles of RNA catalysis and designing valuable tools in biotechnology. High-resolution structures of Psr, extensive structure-function studies, and computation support a mechanism involving one or more catalytic guanosine nucleobases acting as a general base and divalent metal ion-bound water acting as an acid to catalyze RNA 2'-O-transphosphorylation. Yet, for a wide range of pH and metal ion concentrations, the rate of Psr catalysis is too fast to measure manually and the reaction steps that limit catalysis are not well understood. Here, we use stopped-flow fluorescence spectroscopy to evaluate Psr temperature dependence, solvent H/D isotope effects, and divalent metal ion affinity and specificity unconstrained by limitations due to fast kinetics. The results show that Psr catalysis is characterized by small apparent activation enthalpy and entropy changes and minimal transition state H/D fractionation, suggesting that one or more pre-equilibrium steps rather than chemistry is rate limiting. Quantitative analyses of divalent ion dependence confirm that metal aquo ion pKa correlates with higher rates of catalysis independent of differences in ion binding affinity. However, ambiguity regarding the rate-limiting step and similar correlation with related attributes such as ionic radius and hydration free energy complicate a definitive mechanistic interpretation. These new data provide a framework for further interrogation of Psr transition state stabilization and show how thermal instability, metal ion insolubility at optimal pH, and pre-equilibrium steps such as ion binding and folding limit the catalytic power of Psr suggesting potential strategies for further optimization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
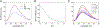


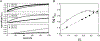







References
-
- Micura R; Höbartner C Fundamental studies of functional nucleic acids: aptamers, riboswitches, ribozymes and DNAzymes. Chem. Soc. Rev 2020, 49, 7331–7353. - PubMed
-
- Balke D; Hieronymus R; Müller S Challenges and Perspectives in Nucleic Acid Enzyme Engineering. Adv. Biochem. Eng. Biotechnol 2020, 170, 21–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

