Rapid recruitment and IFN-I-mediated activation of monocytes dictate focal radiotherapy efficacy
- PMID: 37294749
- PMCID: PMC10340791
- DOI: 10.1126/sciimmunol.add7446
Rapid recruitment and IFN-I-mediated activation of monocytes dictate focal radiotherapy efficacy
Abstract
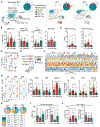
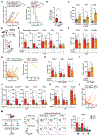
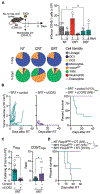
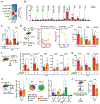
The recruitment of monocytes and their differentiation into immunosuppressive cells is associated with the low efficacy of preclinical nonconformal radiotherapy (RT) for tumors. However, nonconformal RT (non-CRT) does not mimic clinical practice, and little is known about the role of monocytes after RT modes used in patients, such as conformal RT (CRT). Here, we investigated the acute immune response induced by after CRT. Contrary to non-CRT approaches, we found that CRT induces a rapid and robust recruitment of monocytes to the tumor that minimally differentiate into tumor-associated macrophages or dendritic cells but instead up-regulate major histocompatibility complex II and costimulatory molecules. We found that these large numbers of infiltrating monocytes are responsible for activating effector polyfunctional CD8+ tumor-infiltrating lymphocytes that reduce tumor burden. Mechanistically, we show that monocyte-derived type I interferon is pivotal in promoting monocyte accumulation and immunostimulatory function in a positive feedback loop. We also demonstrate that monocyte accumulation in the tumor microenvironment is hindered when RT inadvertently affects healthy tissues, as occurs in non-CRT. Our results unravel the immunostimulatory function of monocytes during clinically relevant modes of RT and demonstrate that limiting the exposure of healthy tissues to radiation has a positive therapeutic effect on the overall antitumor immune response.
Conflict of interest statement
Figures




Comment in
-
Conformal Radiotherapy Efficacy Depends on Monocyte IFN-I Signaling.Cancer Discov. 2023 Aug 4;13(8):1759. doi: 10.1158/2159-8290.CD-RW2023-097. Cancer Discov. 2023. PMID: 37350648
References
-
- Chen H-M, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, Mandeli J, Divino C, Schwartz M, Sung M, Ferris R, Kao J, Wang L-H, Pan P-Y, Ko EC, Chen S-H, Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin. Cancer Res 21, 4073–4085 (2015). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

