Translational imaging of TSPO reveals pronounced innate inflammation in human and murine CD8 T cell-mediated limbic encephalitis
- PMID: 37294768
- PMCID: PMC10256169
- DOI: 10.1126/sciadv.abq7595
Translational imaging of TSPO reveals pronounced innate inflammation in human and murine CD8 T cell-mediated limbic encephalitis
Abstract
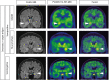
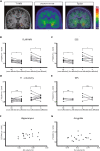
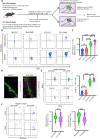
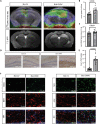
Autoimmune limbic encephalitis (ALE) presents with new-onset mesial temporal lobe seizures, progressive memory disturbance, and other behavioral and cognitive changes. CD8 T cells are considered to play a key role in those cases where autoantibodies (ABs) target intracellular antigens or no ABs were found. Assessment of such patients presents a clinical challenge, and novel noninvasive imaging biomarkers are urgently needed. Here, we demonstrate that visualization of the translocator protein (TSPO) with [18F]DPA-714-PET-MRI reveals pronounced microglia activation and reactive gliosis in the hippocampus and amygdala of patients suspected with CD8 T cell ALE, which correlates with FLAIR-MRI and EEG alterations. Back-translation into a preclinical mouse model of neuronal antigen-specific CD8 T cell-mediated ALE allowed us to corroborate our preliminary clinical findings. These translational data underline the potential of [18F]DPA-714-PET-MRI as a clinical molecular imaging method for the direct assessment of innate immunity in CD8 T cell-mediated ALE.
Figures


References
-
- T. Ravizza, B. Gagliardi, F. Noé, K. Boer, E. Aronica, A. Vezzani, Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 29, 142–160 (2008). - PubMed
-
- C. G. Bien, C. E. Elger, Limbic encephalitis: A cause of temporal lobe epilepsy with onset in adult life. Epilepsy Behav. 10, 529–538 (2007). - PubMed
-
- C. G. Bien, A. Schulze-Bonhage, M. Deckert, H. Urbach, C. Helmstaedter, T. Grunwald, C. Schaller, C. E. Elger, Limbic encephalitis not associated with neoplasm as a cause of temporal lobe epilepsy. Neurology 55, 1823–1828 (2000). - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

