Does providing feedback and guidance on sleep perceptions using sleep wearables improve insomnia? Findings from "Novel Insomnia Treatment Experiment": a randomized controlled trial
- PMID: 37294865
- PMCID: PMC10485571
- DOI: 10.1093/sleep/zsad167
Does providing feedback and guidance on sleep perceptions using sleep wearables improve insomnia? Findings from "Novel Insomnia Treatment Experiment": a randomized controlled trial
Abstract
Study objectives: Insomnia is a disorder diagnosed based on self-reported sleep complaints. Differences between self-reported and sensor-based sleep parameters (sleep-wake state discrepancy) are common but not well-understood in individuals with insomnia. This two-arm, parallel-group, single-blind, superiority randomized-controlled trial examined whether monitoring sleep using wearable devices and providing support for interpretation of sensor-based sleep data improved insomnia symptoms or impacted sleep-wake state discrepancy.
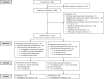
Methods: A total of 113 (age M = 47.53; SD = 14.37, 64.9% female) individuals with significant insomnia symptoms (Insomnia Severity Index(ISI) ≥10) from the community were randomized 1:1 (permuted block randomization) to receive 5 weeks (1) Intervention (n = 57): feedback about sensor-based sleep (Fitbit and EEG headband) with guidance for data interpretation and ongoing monitoring, and (2) Control (n = 56): sleep education and hygiene. Both groups received one individual session and two check-in calls. The ISI (primary outcome), sleep disturbance (SDis), sleep-related impairment (SRI), depression, and anxiety were assessed at baseline and post-intervention.
Results: In total, 103 (91.2%) participants completed the study. Intention-to-treat multiple regression with multiple imputations showed that after controlling for baseline values, compared to the Control group (n = 51), the Intervention group (n = 52) had lower ISI (p = .011, d = 0.51) and SDis (p = .036, d = 0.42) post-intervention, but differences in SRI, depression, anxiety, and sleep-wake state discrepancy parameters (total sleep time, sleep onset latency, and wake after sleep onset) were not meaningful (P-values >.40).
Conclusions: Providing feedback and guidance about sensor-based sleep parameters reduced insomnia severity and sleep disturbance but did not alter sleep-wake state discrepancy in individuals with insomnia more than sleep hygiene and education. The role of sleep wearable devices among individuals with insomnia requires further research.
Clinical trial registration: The Novel Insomnia Treatment Experiment (NITE): the effectiveness of incorporating appropriate guidance for sleep wearables in users with insomnia. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378452, Australia New Zealand Clinical Trials Registry: ACTRN12619001636145.
Keywords: Dreem; Fitbit; Insomnia; Sleep; Sleep–wake estimation; Sleep–wake state discrepancy.
© The Author(s) 2023. Published by Oxford University Press on behalf of Sleep Research Society.
Figures


References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). Washington, DC: American Psychiatric Association Publishing; 2013.
-
- Ancoli-Israel S, Roth T.. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–S353. - PubMed
-
- Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R.. An epidemiological study of insomnia among the Japanese general population. Sleep. 2000;23(1):41–47. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

