Effects of nintedanib in patients with limited cutaneous systemic sclerosis and interstitial lung disease
- PMID: 37294870
- PMCID: PMC10907814
- DOI: 10.1093/rheumatology/kead280
Effects of nintedanib in patients with limited cutaneous systemic sclerosis and interstitial lung disease
Abstract
Objectives: To investigate the course of interstitial lung disease (ILD) and the effects of nintedanib in patients with limited cutaneous systemic sclerosis (lcSSc).
Methods: In the SENSCIS trial, patients with SSc-ILD were randomized to receive nintedanib or placebo. Patients who completed the SENSCIS trial were eligible to enter SENSCIS-ON, in which all patients received open-label nintedanib.
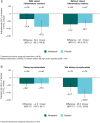
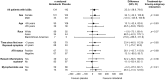
Results: Among 277 patients with lcSSc treated in the SENSCIS trial, the rate (s.e.) of decline in forced vital capacity (FVC; ml/year) over 52 weeks was -74.5 (19.2) in the placebo group and -49.1 (19.8) in the nintedanib group (difference: 25.3 [95% CI -28.9, 79.6]). Among 249 patients with data at week 52, mean (s.e.) change in FVC at week 52 was -86.4 (21.1) ml in the placebo group and -39.1 (22.2) ml in the nintedanib group. Among 183 patients with lcSSc who participated in SENSCIS-ON and had data at week 52, mean (s.e.) change in FVC from baseline to week 52 of SENSCIS-ON was -41.5 (24.0) ml in patients who took placebo in the SENSCIS trial and initiated nintedanib in SENSCIS-ON and -45.1 (19.1) ml in patients who took nintedanib in the SENSCIS trial and continued it in SENSCIS-ON.
Conclusion: Patients with lcSSc may develop progressive fibrosing ILD. By targeting pulmonary fibrosis, nintedanib slows decline in lung function in patients with lcSSc and ILD.
Trial registration: ClinicalTrials.gov (https://clinicaltrials.gov), NCT02597933 and NCT03313180.
Keywords: antifibrotic agents; pulmonary fibrosis; pulmonary function tests; scleroderma; systemic.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Simeón-Aznar CP, Fonollosa-Plá V, Tolosa-Vilella C. et al. Registry of the Spanish network for systemic sclerosis: clinical pattern according to cutaneous subsets and immunological status. Semin Arthritis Rheum 2012;41:789–800. - PubMed
-
- Frantz C, Huscher D, Avouac J. et al. ; EUSTAR Co-authors. Outcomes of limited cutaneous systemic sclerosis patients: results on more than 12,000 patients from the EUSTAR database. Autoimmun Rev 2020;19:102452. - PubMed
-
- Freitas R, Martins P, Dourado E. et al. Clinical features and outcome of 1054 patients with systemic sclerosis: analysis of Reuma.pt/SSc registry. ARP Rheumatol 2022;1:21–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

