Infectious neutrophil deployment is regulated by resolvin D4
- PMID: 37295018
- PMCID: PMC10447623
- DOI: 10.1182/blood.2022019145
Infectious neutrophil deployment is regulated by resolvin D4
Abstract
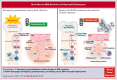
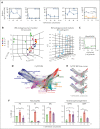
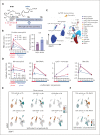
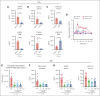
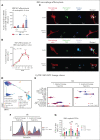
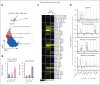
Neutrophils reside in the bone marrow (BM), ready for deployment to sites of injury/infection, initiating inflammation and its resolution. Here, we report that distal infections signal to the BM via resolvins to regulate granulopoiesis and BM neutrophil deployment. Emergency granulopoiesis during peritonitis evoked changes in BM resolvin D1 (RvD1) and BM RvD4. We found that leukotriene B4 stimulates neutrophil deployment. RvD1 and RvD4 each limited neutrophilic infiltration to infections, and differently regulated BM myeloid populations: RvD1 increased reparative monocytes, and RvD4 regulated granulocytes. RvD4 disengaged emergency granulopoiesis, prevented excess BM neutrophil deployment, and acted on granulocyte progenitors. RvD4 also stimulated exudate neutrophil, monocyte, and macrophage phagocytosis, and enhanced bacterial clearance. This mediator accelerated both neutrophil apoptosis and clearance by macrophages, thus expediting the resolution phase of inflammation. RvD4 stimulated phosphorylation of ERK1/2 and STAT3 in human BM-aspirate-derived granulocytes. RvD4 in the 1 to 100 nM range stimulated whole-blood neutrophil phagocytosis of Escherichia coli. RvD4 increased BM macrophage efferocytosis of neutrophils. Together, these results demonstrate the novel functions of resolvins in granulopoiesis and neutrophil deployment, contributing to the resolution of infectious inflammation.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





Comment in
-
Resolvin D4 disengages emergency granulopoiesis.Blood. 2023 Aug 10;142(6):505-507. doi: 10.1182/blood.2023020982. Blood. 2023. PMID: 37561541 No abstract available.
References
-
- Cassatella MA, editor. The Neutrophil: An Emerging Regulator of Inflammatory and Immune Response. Karger; 2003.
-
- Handin RI, Lux SE, Stossel TP. 2nd ed. Lippincott Williams & Wilkins; 2003. Blood: Principles and Practice of Hematology.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

