Transplacental transfer of anti-SARS-CoV-2 neutralizing antibodies in comparison to other pathogens total antibodies
- PMID: 37295035
- PMCID: PMC10212596
- DOI: 10.1016/j.jcv.2023.105495
Transplacental transfer of anti-SARS-CoV-2 neutralizing antibodies in comparison to other pathogens total antibodies
Abstract
Backgrounds: Due to immaturity of their immune system, passive maternal immunization is essential for newborns during their first months of life. Therefore, in the current context of intense circulation of SARS-CoV-2, identifying factors influencing the transfer ratio (TR) of neutralizing antibodies against SARS-CoV-2 (NAb) appears important.
Methods: Our study nested in the COVIPREG cohort (NCT04355234), included mothers who had a SARS-CoV-2 PCR positive during their pregnancy and their newborns. Maternal and neonatal NAb levels were measured with the automated iFlash system.
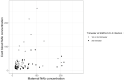
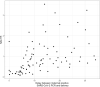
Results: For the 173 mother-infant pairs included in our study, the median gestational age (GA) at delivery was 39.4 weeks of gestation (WG), and 29.7 WG at maternal SARS-CoV-2 infection. Using a multivariate logistic model, having a NAb TR above 1 was positively associated with a longer delay from maternal positive SARS-CoV-2 PCR to delivery (aOR 1.09, 95% CI: 1.03 - 1.17) and with a later GA at delivery (aOR = 1.58, 95% CI: 1.09 - 2.52). It was negatively associated with being a male newborn (aOR 0.21, 95% CI: 0.07 - 0.59). In 3rd trimester SARS-CoV-2 infected mothers, NAb TR was inferior to VZV, toxoplasmosis, CMV, measle and rubella's TR. However, in 1st or 2nd trimester infected mothers, only measle TR was different from NAb TR.
Conclusion: Male newborn of mothers infected by SARS-CoV-2 during their pregnancy appear to have less protection against SARS-CoV-2 in their first months of life than female newborns. Measle TR was superior to NAb TR even in case of 1st or 2nd trimester maternal SARS-CoV-2 infection. Future studies are needed to investigate possible differences in transmission of NAb following infection vs vaccination and its impact on TR.
Keywords: Neutralizing antibodies; Newborn; Pregnancy; SARS-CoV-2; Transplacental transmission.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The author, Diane Brebant, declare receiving a grant from the Groupe de Recherche sur les Infections pendant la Grossesse (GRIG) for this work.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

