Mechanism of ribosome-associated mRNA degradation during tubulin autoregulation
- PMID: 37295431
- PMCID: PMC10403363
- DOI: 10.1016/j.molcel.2023.05.020
Mechanism of ribosome-associated mRNA degradation during tubulin autoregulation
Abstract
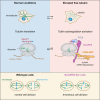
Microtubules play crucial roles in cellular architecture, intracellular transport, and mitosis. The availability of free tubulin subunits affects polymerization dynamics and microtubule function. When cells sense excess free tubulin, they trigger degradation of the encoding mRNAs, which requires recognition of the nascent polypeptide by the tubulin-specific ribosome-binding factor TTC5. How TTC5 initiates the decay of tubulin mRNAs is unknown. Here, our biochemical and structural analysis reveals that TTC5 recruits the poorly studied protein SCAPER to the ribosome. SCAPER, in turn, engages the CCR4-NOT deadenylase complex through its CNOT11 subunit to trigger tubulin mRNA decay. SCAPER mutants that cause intellectual disability and retinitis pigmentosa in humans are impaired in CCR4-NOT recruitment, tubulin mRNA degradation, and microtubule-dependent chromosome segregation. Our findings demonstrate how recognition of a nascent polypeptide on the ribosome is physically linked to mRNA decay factors via a relay of protein-protein interactions, providing a paradigm for specificity in cytoplasmic gene regulation.
Keywords: CCR4-NOT complex; RNA degradation; co-translational regulation; microtubules; ribosome; tubulin.
Copyright © 2023 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.A.P. is an inventor on a patent filed by the Medical Research Council for all-gold EM supports, licensed to Quantifoil under the trademark UltrAuFoil. L.A.P. and R.S.H. are on the advisory board for Molecular Cell.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

