Implications of the Approval of Lecanemab for Alzheimer Disease Patient Care: Incremental Step or Paradigm Shift?
- PMID: 37295957
- PMCID: PMC10573150
- DOI: 10.1212/WNL.0000000000207438
Implications of the Approval of Lecanemab for Alzheimer Disease Patient Care: Incremental Step or Paradigm Shift?
Abstract
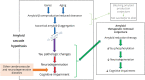
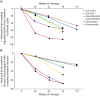
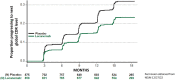
The amyloid cascade model of the pathogenesis of Alzheimer disease (AD) is well supported in observational studies. Its therapeutic corollary asserts that removal of amyloid-β peptide ("amyloid") would provide clinical benefits. After 2 decades of pursuing the strategy of amyloid removal without success, clinical trials of the antiamyloid monoclonal antibody (AAMA) donanemab and a phase 3 clinical trial of lecanemab have reported clinical benefits linked to amyloid removal. Lecanemab (trade name, Leqembi) is the first with published phase 3 trial results. When administered through IV every 2 weeks to patients with elevated brain amyloid and mild cognitive impairment or mild dementia, lecanemab delayed cognitive and functional worsening by approximately 5 months in an 18-month double-blind, placebo-controlled trial. The trial was well conducted, and the results favoring lecanemab were internally consistent. The demonstration that lecanemab treatment delayed clinical progression in persons with mild symptoms due to AD is a major conceptual achievement, but a better appreciation of the magnitude and durability of benefits for individual patients will require extended observations from clinical practice settings. Amyloid-related imaging abnormalities (ARIA) that were largely asymptomatic occurred in approximately 20%, slightly more than half of which were attributable to treatment and the rest to underlying AD-related amyloid angiopathy. Persons who were homozygous for the APOE ε4 allele had greater ARIA risks. Hemorrhagic complications with longer-term lecanemab use need to be better understood. Administration of lecanemab will place unprecedented pressures on dementia care personnel and infrastructure, both of which need to grow exponentially to meet the challenge.
© 2023 American Academy of Neurology.
Conflict of interest statement
D.S. Knopman serves on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer Network Treatment Unit study. He served on a Data Safety monitoring Board for a tau therapeutic for Biogen (until 2021) but received no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals, and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health, Biovie, and Alzeca Biosciences but receives no personal compensation. He attended an Eisai advisory board meeting for lecanemab on December 2, 2022, but received no compensation directly or indirectly. He receives funding from the NIH. L. Hershey serves as an Associate Editor for the journal Neurology. She prepares annual updates on memory loss, pre-MCI, vascular cognitive impairment and other topics for MedLink Neurology. Go to
Figures

Similar articles
-
Lecanemab: Appropriate Use Recommendations.J Prev Alzheimers Dis. 2023;10(3):362-377. doi: 10.14283/jpad.2023.30. J Prev Alzheimers Dis. 2023. PMID: 37357276 Free PMC article.
-
Updated safety results from phase 3 lecanemab study in early Alzheimer's disease.Alzheimers Res Ther. 2024 May 10;16(1):105. doi: 10.1186/s13195-024-01441-8. Alzheimers Res Ther. 2024. PMID: 38730496 Free PMC article. Clinical Trial.
-
Lecanemab Therapy and APOE Genotype.2024 Aug 12 [updated 2024 Nov 25]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. 2024 Aug 12 [updated 2024 Nov 25]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kattman BL, Malheiro AJ, editors. Medical Genetics Summaries [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2012–. PMID: 39141762 Free Books & Documents. Review.
-
A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Aβ protofibril antibody.Alzheimers Res Ther. 2021 Apr 17;13(1):80. doi: 10.1186/s13195-021-00813-8. Alzheimers Res Ther. 2021. PMID: 33865446 Free PMC article. Clinical Trial.
-
Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer's disease: A systematic review.Int J Immunopathol Pharmacol. 2023 Jan-Dec;37:3946320231209839. doi: 10.1177/03946320231209839. Int J Immunopathol Pharmacol. 2023. PMID: 37902139 Free PMC article.
Cited by
-
Synthesis and Enzymatic Evaluation of a Small Library of Substituted Phenylsulfonamido-Alkyl Sulfamates towards Carbonic Anhydrase II.Molecules. 2024 Jun 25;29(13):3015. doi: 10.3390/molecules29133015. Molecules. 2024. PMID: 38998967 Free PMC article.
-
Alzheimer's Disease Immunotherapy and Mimetic Peptide Design for Drug Development: Mutation Screening, Molecular Dynamics, and a Quantum Biochemistry Approach Focusing on Aducanumab::Aβ2-7 Binding Affinity.ACS Chem Neurosci. 2024 Oct 2;15(19):3543-3562. doi: 10.1021/acschemneuro.4c00453. Epub 2024 Sep 20. ACS Chem Neurosci. 2024. PMID: 39302203 Free PMC article.
-
Precision diagnosis of cognitive impairment due to Alzheimer's disease for therapeutic interventions.Alzheimers Dement. 2025 Jan;21(1):e14043. doi: 10.1002/alz.14043. Epub 2024 Dec 24. Alzheimers Dement. 2025. PMID: 39718338 Free PMC article. Review.
-
Contribution of Modifiable Midlife and Late-Life Vascular Risk Factors to Incident Dementia.JAMA Neurol. 2025 Jul 1;82(7):644-654. doi: 10.1001/jamaneurol.2025.1495. JAMA Neurol. 2025. PMID: 40455489
-
Evaluation of Amyloid Removal as a Surrogate for Cognitive Decline: Pilot Analysis in Individual-Level Data from the A4 Study of Solanezumab.medRxiv [Preprint]. 2025 Jul 22:2025.07.21.25331942. doi: 10.1101/2025.07.21.25331942. medRxiv. 2025. PMID: 40778134 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
