IGFBP1 Sustains Cell Survival during Spatially-Confined Migration and Promotes Tumor Metastasis
- PMID: 37296072
- PMCID: PMC10375137
- DOI: 10.1002/advs.202206540
IGFBP1 Sustains Cell Survival during Spatially-Confined Migration and Promotes Tumor Metastasis
Abstract
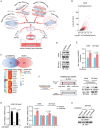
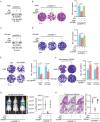
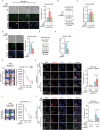
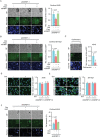
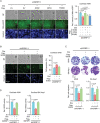
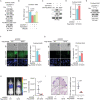
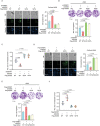
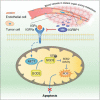
Cell migration is a pivotal step in metastatic process, which requires cancer cells to navigate a complex spatially-confined environment, including tracks within blood vessels and in the vasculature of target organs. Here it is shown that during spatially-confined migration, the expression of insulin-like growth factor-binding protein 1 (IGFBP1) is upregulated in tumor cells. Secreted IGFBP1 inhibits AKT1-mediated phosphorylation of mitochondrial superoxide dismutase (SOD2) serine (S) 27 and enhances SOD2 activity. Enhanced SOD2 attenuates mitochondrial reactive oxygen species (ROS) accumulation in confined cells, which supports tumor cell survival in blood vessels of lung tissues, thereby accelerating tumor metastasis in mice. The levels of blood IGFBP1 correlate with metastatic recurrence of lung cancer patients. This finding reveals a unique mechanism by which IGFBP1 sustains cell survival during confined migration by enhancing mitochondrial ROS detoxification, thereby promoting tumor metastasis.
Keywords: confined migration; insulin-like growth factor-binding protein 1; mitochondrial reactive oxygen species; superoxide dismutase; tumor metastasis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Emodin Increases Expression of Insulin-Like Growth Factor Binding Protein 1 through Activation of MEK/ERK/AMPKα and Interaction of PPARγ and Sp1 in Lung Cancer.Cell Physiol Biochem. 2017;41(1):339-357. doi: 10.1159/000456281. Epub 2017 Jan 26. Cell Physiol Biochem. 2017. PMID: 28214826
-
MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells.Oncogene. 2017 Oct 19;36(42):5897-5909. doi: 10.1038/onc.2017.167. Epub 2017 Jun 26. Oncogene. 2017. PMID: 28650465
-
p66ShcA functions as a contextual promoter of breast cancer metastasis.Breast Cancer Res. 2020 Jan 15;22(1):7. doi: 10.1186/s13058-020-1245-6. Breast Cancer Res. 2020. PMID: 31941526 Free PMC article.
-
Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis.Biochim Biophys Acta Bioenerg. 2017 Aug;1858(8):628-632. doi: 10.1016/j.bbabio.2017.01.006. Epub 2017 Jan 11. Biochim Biophys Acta Bioenerg. 2017. PMID: 28087256 Free PMC article. Review.
-
The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance.J Exp Clin Cancer Res. 2018 Mar 16;37(1):61. doi: 10.1186/s13046-018-0728-0. J Exp Clin Cancer Res. 2018. PMID: 29548337 Free PMC article. Review.
Cited by
-
Defining lung adenocarcinoma subtypes with glucocorticoid-related genes and constructing a prognostic index for immunotherapy guidance.J Thorac Dis. 2025 Apr 30;17(4):1888-1905. doi: 10.21037/jtd-24-1083. Epub 2025 Apr 28. J Thorac Dis. 2025. PMID: 40400930 Free PMC article.
-
Banxia Xiexin Tang attenuates high glucose-induced hepatocyte injury by activating SOD2 to scavenge ROS via PGC-1α/IGFBP1.3 Biotech. 2024 Sep;14(9):216. doi: 10.1007/s13205-024-04060-0. Epub 2024 Aug 28. 3 Biotech. 2024. PMID: 39220826
-
Machine learning-based prognostic model of lactylation-related genes for predicting prognosis and immune infiltration in patients with lung adenocarcinoma.Cancer Cell Int. 2024 Dec 18;24(1):400. doi: 10.1186/s12935-024-03592-y. Cancer Cell Int. 2024. PMID: 39696439 Free PMC article.
-
Deciphering the multidimensional impact of IGFBP1 expression on cancer prognosis, genetic alterations, and cellular functionality: A comprehensive Pan-cancer analysis.Heliyon. 2024 Sep 6;10(18):e37402. doi: 10.1016/j.heliyon.2024.e37402. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309809 Free PMC article.
-
The systematic analysis of genes related to butyrate metabolism suggests that CDKN3 could serve as a promising therapeutic target for lung adenocarcinoma treatment.BMC Cancer. 2025 Jan 13;25(1):69. doi: 10.1186/s12885-024-13409-w. BMC Cancer. 2025. PMID: 39806313 Free PMC article.
References
-
- Gupta G. P., Massague J., Cell 2006, 127, 679. - PubMed
-
- Chaffer C. L., Weinberg R. A., Science 2011, 331, 1559. - PubMed
-
- Muller A., Muller S., Nasufovic V., Arndt H. D., Pompe T., Integr. Biol. 2019, 11, 175. - PubMed
-
- Alexander S., Koehl G. E., Hirschberg M., Geissler E. K., Friedl P., Histochem. Cell Biol. 2008, 130, 1147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
