Transplanting Human Neural Stem Cells with ≈50% Reduction of SOX9 Gene Dosage Promotes Tissue Repair and Functional Recovery from Severe Spinal Cord Injury
- PMID: 37296073
- PMCID: PMC10369238
- DOI: 10.1002/advs.202205804
Transplanting Human Neural Stem Cells with ≈50% Reduction of SOX9 Gene Dosage Promotes Tissue Repair and Functional Recovery from Severe Spinal Cord Injury
Abstract
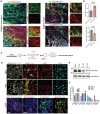
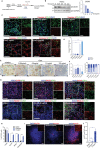
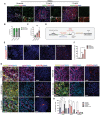
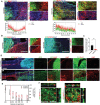
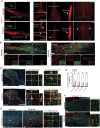
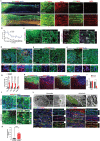
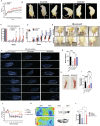
Neural stem cells (NSCs) derived from human pluripotent stem cells (hPSCs) are considered a major cell source for reconstructing damaged neural circuitry and enabling axonal regeneration. However, the microenvironment at the site of spinal cord injury (SCI) and inadequate intrinsic factors limit the therapeutic potential of transplanted NSCs. Here, it is shown that half dose of SOX9 in hPSCs-derived NSCs (hNSCs) results in robust neuronal differentiation bias toward motor neuron lineage. The enhanced neurogenic potency is partly attributed to the reduction of glycolysis. These neurogenic and metabolic properties retain after transplantation of hNSCs with reduced SOX9 expression in a contusive SCI rat model without the need for growth factor-enriched matrices. Importantly, the grafts exhibit excellent integration properties, predominantly differentiate into motor neurons, reduce glial scar matrix accumulation to facilitate long-distance axon growth and neuronal connectivity with the host as well as dramatically improve locomotor and somatosensory function in recipient animals. These results demonstrate that hNSCs with half SOX9 gene dosage can overcome extrinsic and intrinsic barriers, representing a powerful therapeutic potential for transplantation treatments for SCI.
Keywords: SOX9; human neural stem cells; motor neurons; pluripotent stem cells; spinal cord injury.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mash-1 modified neural stem cells transplantation promotes neural stem cells differentiation into neurons to further improve locomotor functional recovery in spinal cord injury rats.Gene. 2021 May 20;781:145528. doi: 10.1016/j.gene.2021.145528. Epub 2021 Feb 22. Gene. 2021. PMID: 33631250
-
Effects of the Post-Spinal Cord Injury Microenvironment on the Differentiation Capacity of Human Neural Stem Cells Derived from Induced Pluripotent Stem Cells.Cell Transplant. 2016 Oct;25(10):1833-1852. doi: 10.3727/096368916X691312. Cell Transplant. 2016. PMID: 27075820
-
Suppressing CSPG/LAR/PTPσ Axis Facilitates Neuronal Replacement and Synaptogenesis by Human Neural Precursor Grafts and Improves Recovery after Spinal Cord Injury.J Neurosci. 2022 Apr 13;42(15):3096-3121. doi: 10.1523/JNEUROSCI.2177-21.2022. Epub 2022 Mar 7. J Neurosci. 2022. PMID: 35256527 Free PMC article.
-
Human stem cell-derived neurons and neural circuitry therapeutics: Next frontier in spinal cord injury repair.Exp Biol Med (Maywood). 2022 Dec;247(23):2142-2151. doi: 10.1177/15353702221114099. Epub 2022 Aug 16. Exp Biol Med (Maywood). 2022. PMID: 35974701 Free PMC article. Review.
-
Direct neuronal differentiation of neural stem cells for spinal cord injury repair.Stem Cells. 2021 Aug;39(8):1025-1032. doi: 10.1002/stem.3366. Epub 2021 Mar 5. Stem Cells. 2021. PMID: 33657255 Review.
Cited by
-
Biological engineering approaches for modulating the pathological microenvironment and promoting axonal regeneration after spinal cord injury.Front Neurosci. 2025 May 12;19:1574763. doi: 10.3389/fnins.2025.1574763. eCollection 2025. Front Neurosci. 2025. PMID: 40421136 Free PMC article. Review.
-
Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go.Biomedicines. 2025 May 21;13(5):1262. doi: 10.3390/biomedicines13051262. Biomedicines. 2025. PMID: 40427089 Free PMC article. Review.
-
Proteomics and lipidomics of human umbilical cord mesenchymal stem cells exposed to ionizing radiation.Eur J Med Res. 2025 Apr 28;30(1):340. doi: 10.1186/s40001-025-02578-4. Eur J Med Res. 2025. PMID: 40296080 Free PMC article.
-
Recovery of Forearm and Fine Digit Function After Chronic Spinal Cord Injury by Simultaneous Blockade of Inhibitory Matrix Chondroitin Sulfate Proteoglycan Production and the Receptor PTPσ.J Neurotrauma. 2023 Dec;40(23-24):2500-2521. doi: 10.1089/neu.2023.0117. Epub 2023 Oct 11. J Neurotrauma. 2023. PMID: 37606910 Free PMC article.
-
Exosomes derived from CD271+CD56+ bone marrow mesenchymal stem cell subpopoulation identified by single-cell RNA sequencing promote axon regeneration after spinal cord injury.Theranostics. 2024 Jan 1;14(2):510-527. doi: 10.7150/thno.89008. eCollection 2024. Theranostics. 2024. PMID: 38169566 Free PMC article.
References
-
- a) Silver J., Miller J. H., Nat. Rev. Neurosci. 2004, 5, 146; - PubMed
- b) Hara M., Kobayakawa K., Ohkawa Y., Kumamaru H., Yokota K., Saito T., Kijima K., Yoshizaki S., Harimaya K., Nakashima Y., Okada S., Nat. Med. 2017, 23, 818; - PubMed
- c) Bradbury E. J., Burnside E. R., Nat. Commun. 2019, 10, 3879. - PMC - PubMed
-
- a) Yokota K., Kobayakawa K., Kubota K., Miyawaki A., Okano H., Ohkawa Y., Iwamoto Y., Okada S., Stem Cell Rep. 2015, 5, 264; - PMC - PubMed
- b) Dell'Anno M. T., Wang X., Onorati M., Li M., Talpo F., Sekine Y., Ma S., Liu F., Cafferty W. B. J., Sestan N., Strittmatter S. M., Nat. Commun. 2018, 9, 3419; - PMC - PubMed
- c) Kumamaru H., Kadoya K., Adler A. F., Takashima Y., Graham L., Coppola G., Tuszynski M. H., Nat. Methods 2018, 15, 723; - PubMed
- d) Rosenzweig E. S., Brock J. H., Lu P., Kumamaru H., Salegio E. A., Kadoya K., Weber J. L., Liang J. J., Moseanko R., Hawbecker S., Huie J. R., Havton L. A., Nout‐Lomas Y. S., Ferguson A. R., Beattie M. S., Bresnahan J. C., Tuszynski M. H., Nat. Med. 2018, 24, 484. - PMC - PubMed
-
- a) Martino G., Pluchino S., Nat. Rev. Neurosci. 2006, 7, 395; - PubMed
- b) Yoshizaki S., Tamaru T., Hara M., Kijima K., Tanaka M., Konno D. J., Matsumoto Y., Nakashima Y., Okada S., J Neuroinflammation 2021, 18, 12; - PMC - PubMed
- c) Khazaei M., Ahuja C. S., Nakashima H., Nagoshi N., Li L., Wang J., Chio J., Badner A., Seligman D., Ichise A., Shibata S., Fehlings M. G., Sci. Transl. Med. 2020, 12, 525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
