Histone H2A Lys130 acetylation epigenetically regulates androgen production in prostate cancer
- PMID: 37296155
- PMCID: PMC10256812
- DOI: 10.1038/s41467-023-38887-7
Histone H2A Lys130 acetylation epigenetically regulates androgen production in prostate cancer
Erratum in
-
Author Correction: Histone H2A Lys130 acetylation epigenetically regulates androgen production in prostate cancer.Nat Commun. 2023 Sep 27;14(1):6022. doi: 10.1038/s41467-023-41810-9. Nat Commun. 2023. PMID: 37758702 Free PMC article. No abstract available.
Abstract
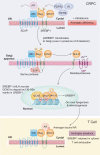
The testicular androgen biosynthesis is well understood, however, how cancer cells gauge dwindling androgen to dexterously initiate its de novo synthesis remained elusive. We uncover dual-phosphorylated form of sterol regulatory element-binding protein 1 (SREBF1), pY673/951-SREBF1 that acts as an androgen sensor, and dissociates from androgen receptor (AR) in androgen deficient environment, followed by nuclear translocation. SREBF1 recruits KAT2A/GCN5 to deposit epigenetic marks, histone H2A Lys130-acetylation (H2A-K130ac) in SREBF1, reigniting de novo lipogenesis & steroidogenesis. Androgen prevents SREBF1 nuclear translocation, promoting T cell exhaustion. Nuclear SREBF1 and H2A-K130ac levels are significantly increased and directly correlated with late-stage prostate cancer, reversal of which sensitizes castration-resistant prostate cancer (CRPC) to androgen synthesis inhibitor, Abiraterone. Further, we identify a distinct CRPC lipid signature resembling lipid profile of prostate cancer in African American (AA) men. Overall, pY-SREBF1/H2A-K130ac signaling explains cancer sex bias and reveal synchronous inhibition of KAT2A and Tyr-kinases as an effective therapeutic strategy.
© 2023. The Author(s).
Conflict of interest statement
A patent “Inhibitors of ACK1/TNK2 Tyrosine Kinase” (patent no. 9,850,216; 10,017,478 and 10,336,734) covers (R)-9b compound. NPM and KM are named as inventors. These patents have been licensed by TechnoGenesys Inc. KM and NPM are co-founders of TechnoGenesys Inc., own stocks, and serve as consultants for TechnoGenesys Inc. The other authors declare no potential conflicts of interest..
Figures






References
-
- Locke JA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. - PubMed
-
- Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin. Cancer Res. 2004;10:7121–7126. - PubMed
-
- Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 2005;11:4653–4657. - PubMed
-
- Nishiyama T, Ikarashi T, Hashimoto Y, Wako K, Takahashi K. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J. Urol. 2007;178:1282–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

