Loss of SFXN1 mitigates lipotoxicity and predicts poor outcome in non-viral hepatocellular carcinoma
- PMID: 37296228
- PMCID: PMC10256799
- DOI: 10.1038/s41598-023-36660-w
Loss of SFXN1 mitigates lipotoxicity and predicts poor outcome in non-viral hepatocellular carcinoma
Abstract
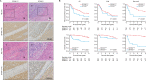
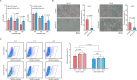
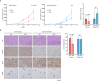
Hepatocellular carcinoma (HCC) imposes a huge global burden, arising from various etiological factors such as hepatitis virus infection and metabolic syndrome. While prophylactic vaccination and antiviral treatment have decreased the incidence of viral HCC, the growing prevalence of metabolic syndrome has led to an increase in non-viral HCC. To identify genes downregulated and specifically associated with unfavorable outcome in non-viral HCC cases, screening analysis was conducted using publically available transcriptome data. Among top 500 genes meeting the criteria, which were involved in lipid metabolism and mitochondrial function, a serine transporter located on inner mitochondrial membrane SFXN1 was highlighted. SFXN1 protein expression was significantly reduced in 33 of 105 HCC tissue samples, and correlated to recurrence-free and overall survival only in non-viral HCC. Human HCC cells with SFXN1 knockout (KO) displayed higher cell viability, lower fat intake and diminished reactive oxygen species (ROS) production in response to palmitate administration. In a subcutaneous transplantation mouse model, high-fat diet feeding attenuated tumorigenic potential in the control cells, but not in the SFXN1-KO cells. In summary, loss of SFXN1 expression suppresses lipid accumulation and ROS generation, preventing toxic effects from fat overload in non-viral HCC, and predicts clinical outcome of non-viral HCC patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Fitzmaurice C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

