The Effects of NLY01, a Novel Glucagon-Like Peptide-1 Receptor Agonist, on Cuprizone-Induced Demyelination and Remyelination: Challenges and Future Perspectives
- PMID: 37296356
- PMCID: PMC10457267
- DOI: 10.1007/s13311-023-01390-4
The Effects of NLY01, a Novel Glucagon-Like Peptide-1 Receptor Agonist, on Cuprizone-Induced Demyelination and Remyelination: Challenges and Future Perspectives
Abstract
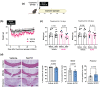
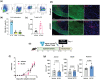
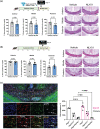
Recent evidence suggests that the glucagon-like peptide-1 receptor (GLP-1R) agonists have neuroprotective activities in the CNS in animal models of Parkinson's disease, Alzheimer's disease, and multiple sclerosis (MS). This study aimed to investigate whether a novel long-acting GLP-1R agonist, NLY01, could limit demyelination or improve remyelination as occurs in MS using the cuprizone (CPZ) mouse model. Herein, we assessed the expression of GLP-1R on oligodendrocytes in vitro and found that mature oligodendrocytes (Olig2+PDGFRa-) express GLP-1R. We further confirmed this observation in the brain by immunohistochemistry and found that Olig2+CC1+ cells express GLP-1R. We next administered NLY01 twice per week to C57B6 mice while on CPZ chow diet and found that NLY01 significantly reduced demyelination with greater weight loss than vehicle-treated controls. Because GLP-1R agonists are known to have anorexigenic effect, we then administered CPZ by oral gavage and treated the mice with NLY01 or vehicle to ensure the dose consistency of CPZ ingestion among mice. Using this modified approach, NLY01 was no longer effective in reducing demyelination of the corpus callosum (CC). We next sought to examine the effects of NLY01 treatment on remyelination after CPZ intoxication and during the recovery period using an adoptive transfer-CPZ (AT-CPZ) model. We found no significant differences between the NLY01 and vehicle groups in the amount of myelin or the number of mature oligodendrocytes in the CC. In summary, despite the promising anti-inflammatory and neuroprotective effects of GLP-1R agonists that have been previously described, our experiments provided no evidence to support a beneficial effect of NLY01 on limiting demyelination or enhancing remyelination. This information may be useful in selecting proper outcome measures in clinical trials of this promising class of drugs in MS.
Keywords: Cuprizone; GLP-1R; MS; Myelin; NLY01; Oligodendrocytes.
© 2023. The American Society for Experimental Neurotherapeutics, Inc.
Conflict of interest statement
None.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

