Effect of propranolol and clonidine after severe traumatic brain injury: a pilot randomized clinical trial
- PMID: 37296432
- PMCID: PMC10251526
- DOI: 10.1186/s13054-023-04479-6
Effect of propranolol and clonidine after severe traumatic brain injury: a pilot randomized clinical trial
Abstract
Objective: To evaluate the safety, feasibility, and efficacy of combined adrenergic blockade with propranolol and clonidine in patients with severe traumatic brain injury (TBI).
Background: Administration of adrenergic blockade after severe TBI is common. To date, no prospective trial has rigorously evaluated this common therapy for benefit.
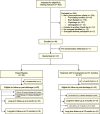
Methods: This phase II, single-center, double-blinded, pilot randomized placebo-controlled trial included patients aged 16-64 years with severe TBI (intracranial hemorrhage and Glasgow Coma Scale score ≤ 8) within 24 h of ICU admission. Patients received propranolol and clonidine or double placebo for 7 days. The primary outcome was ventilator-free days (VFDs) at 28 days. Secondary outcomes included catecholamine levels, hospital length of stay, mortality, and long-term functional status. A planned futility assessment was performed mid-study.
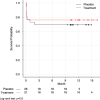
Results: Dose compliance was 99%, blinding was intact, and no open-label agents were used. No treatment patient experienced dysrhythmia, myocardial infarction, or cardiac arrest. The study was stopped for futility after enrolling 47 patients (26 placebo, 21 treatment), per a priori stopping rules. There was no significant difference in VFDs between treatment and control groups [0.3 days, 95% CI (- 5.4, 5.8), p = 1.0]. Other than improvement of features related to sympathetic hyperactivity (mean difference in Clinical Features Scale (CFS) 1.7 points, CI (0.4, 2.9), p = 0.012), there were no between-group differences in the secondary outcomes.
Conclusion: Despite the safety and feasibility of adrenergic blockade with propranolol and clonidine after severe TBI, the intervention did not alter the VFD outcome. Given the widespread use of these agents in TBI care, a multi-center investigation is warranted to determine whether adrenergic blockade is of therapeutic benefit in patients with severe TBI. Trial Registration Number NCT01322048.
Keywords: Adrenergic blockade; Critical care; Paroxysmal sympathetic hyperactivity; Traumatic brain injury.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Pfizer, Inc. (previously Hospira Inc.) has provided unrelated research support to PPP. Haemonetics, Inc. has provided unrelated research support to MBP. For the remaining authors, no competing interests were declared.
Figures
References
-
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. - PubMed
-
- National Academies of Sciences E, and Medicine 2022: Traumatic Brain Injury: A Roadmap for Accelerating Progress. In. Washington, DC; 2022. - PubMed
-
- Traumatic Brain Injury Related Emergency Department Visits, Hospitalizations and Deaths [https://www.cdc.gov/traumaticbraininjury/data/tbi-edhd.html].
-
- Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma. 2007;24(2):232–238. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical