Testis cell pyroptosis mediated by CASP1 and CASP4: possible sertoli cell-only syndrome pathogenesis
- PMID: 37296437
- PMCID: PMC10250861
- DOI: 10.1186/s12958-023-01101-w
Testis cell pyroptosis mediated by CASP1 and CASP4: possible sertoli cell-only syndrome pathogenesis
Abstract
Background: Sertoli cell-only syndrome (SCOS) is the most serious pathological type of non-obstructive azoospermia. Recently, several genes related to SCOS have been identified, including FANCM, TEX14, NR5A1, NANOS2, PLK4, WNK3, and FANCA, but they cannot fully explain the pathogenesis of SCOS. This study attempted to explain spermatogenesis dysfunction in SCOS through testicular tissue RNA sequencing and to provide new targets for SCOS diagnosis and therapy.
Methods: We analyzed differentially expressed genes (DEGs) based on RNA sequencing of nine patients with SCOS and three patients with obstructive azoospermia and normal spermatogenesis. We further explored the identified genes using ELISA and immunohistochemistry.
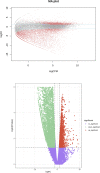
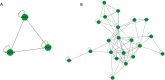
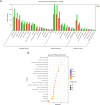
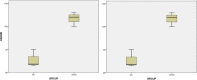
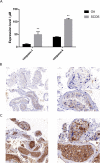
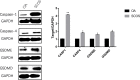
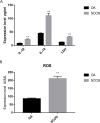
Results: In total, 9406 DEGs were expressed (Log2|FC|≥ 1; adjusted P value < 0.05) in SCOS samples, and 21 hub genes were identified. Three upregulated core genes were found, including CASP4, CASP1, and PLA2G4A. Thus, we hypothesized that testis cell pyroptosis mediated by CASP1 and CASP4 might be involved in SCOS occurrence and development. ELISA verified that CASP1 and CASP4 activities in the testes of patients with SCOS were significantly higher than those in patients with normal spermatogenesis. Immunohistochemical results showed that CASP1 and CASP4 in the normal spermatogenesis group were mainly expressed in the nuclei of spermatogenic, Sertoli, and interstitial cells. CASP1 and CASP4 in the SCOS group were mainly expressed in the nuclei of Sertoli and interstitial cells because of the loss of spermatogonia and spermatocytes. CASP1 and CASP4 expression levels in the testes of patients with SCOS were significantly higher than those in patients with normal spermatogenisis. Furthermore, the pyroptosis-related proteins GSDMD and GSDME in the testes of patients with SCOS were also significantly higher than those in control patients. ELISA also showed that inflammatory factors (IL-1 β, IL-18, LDH, and ROS) were significantly increased in the SCOS group.
Conclusions: For the first time, we found that cell pyroptosis-related genes and key markers were significantly increased in the testes of patients with SCOS. We also observed many inflammatory and oxidative stress reactions in SCOS. Thus, we propose that testis cell pyroptosis mediated by CASP1 and CASP4 could participate in SCOS occurrence and development.
Keywords: CASP1; CASP4; Pyroptosis; Sertoli cell-only syndrome (SCOS).
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Niederberger C. Re: common variants in mismatch repair genes associated with increased risk of sperm DNA damage and male infertility. J Urol. 2013;189(1):255. - PubMed
-
- Ma M, Yang S, Zhang Z, Li P, Gong Y, Liu L, Zhu Y, Tian R, Liu Y, Wang X, Liu F, He L, Liu Y, Yang H, Li Z, He Z. Sertoli cells from non-obstructive azoospermia and obstructive azoospermia patients show distinct morphology, Raman spectrum and biochemical phenotype. Hum Reprod. 2013;28(7):1863–1873. doi: 10.1093/humrep/det068. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

