Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro
- PMID: 37296657
- PMCID: PMC10252311
- DOI: 10.3390/cells12111537
Short-Term Exposure to Bisphenol A Does Not Impact Gonadal Cell Steroidogenesis In Vitro
Abstract
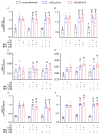
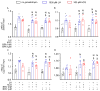
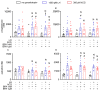
Bisphenol A (BPA) is a ubiquitous, synthetic chemical proven to induce reproductive disorders in both men and women. The available studies investigated the effects of BPA on male and female steroidogenesis following long-term exposure to the compound at relatively high environmental concentrations. However, the impact of short-term exposure to BPA on reproduction is poorly studied. We evaluated if 8 and 24 h exposure to 1 nM and 1 µM BPA perturbs luteinizing hormone/choriogonadotropin (LH/hCG)-mediated signalling in two steroidogenic cell models, i.e., the mouse tumour Leydig cell line mLTC1, and human primary granulosa lutein cells (hGLC). Cell signalling studies were performed using a homogeneous time-resolved fluorescence (HTRF) assay and Western blotting, while gene expression analysis was carried out using real-time PCR. Immunostainings and an immunoassay were used for intracellular protein expression and steroidogenesis analyses, respectively. The presence of BPA leads to no significant changes in gonadotropin-induced cAMP accumulation, alongside phosphorylation of downstream molecules, such as ERK1/2, CREB and p38 MAPK, in both the cell models. BPA did not impact STARD1, CYP11A1 and CYP19A1 gene expression in hGLC, nor Stard1 and Cyp17a1 expression in mLTC1 treated with LH/hCG. Additionally, the StAR protein expression was unchanged upon exposure to BPA. Progesterone and oestradiol levels in the culture medium, measured by hGLC, as well as the testosterone and progesterone levels in the culture medium, measured by mLTC1, did not change in the presence of BPA combined with LH/hCG. These data suggest that short-term exposure to environmental concentrations of BPA does not compromise the LH/hCG-induced steroidogenic potential of either human granulosa or mouse Leydig cells.
Keywords: Bisphenol A (BPA); LH; hCG; ovary; steroidogenesis; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Peretz J., Vrooman L., Ricke W.A., Hunt P.A., Ehrlich S., Hauser R., Padmanabhan V., Taylor H.S., Swan S.H., Vandevoort C.A., et al. Bisphenol A and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

