Lot-to-Lot Variance in Immunoassays-Causes, Consequences, and Solutions
- PMID: 37296687
- PMCID: PMC10252387
- DOI: 10.3390/diagnostics13111835
Lot-to-Lot Variance in Immunoassays-Causes, Consequences, and Solutions
Abstract
Immunoassays, which have gained popularity in clinical practice and modern biomedical research, play an increasingly important role in quantifying various analytes in biological samples. Despite their high sensitivity and specificity, as well as their ability to analyze multiple samples in a single run, immunoassays are plagued by the problem of lot-to-lot variance (LTLV). LTLV negatively affects assay accuracy, precision, and specificity, leading to considerable uncertainty in reported results. Therefore, maintaining consistency in technical performance over time presents a challenge in reproducing immunoassays. In this article, we share our two-decade-long experience and delve into the reasons for and locations of LTLV, as well as explore methods to mitigate its effects. Our investigation identifies potential contributing factors, including quality fluctuation in critical raw materials and deviations in manufacturing processes. These findings offer valuable insights to developers and researchers working with immunoassays, emphasizing the importance of considering lot-to-lot variance in assay development and application.
Keywords: critical quality attributes; immunoassay; lot-to-lot variance; quality control.
Conflict of interest statement
Y.L., M.P., L.A., S.S., A.-C.B.-J. and M.K. are full-time employees of Nordic Bioscience. The authors declare no conflict of interest.
Figures

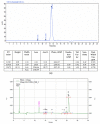

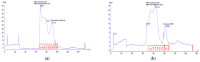

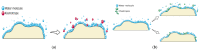
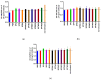
References
-
- Böttcher S., van der Velden V.H.J., Villamor N., Ritgen M., Flores-Montero J., Escobar H.M., Kalina T., Brüggemann M., Grigore G., Martin-Ayuso M., et al. Lot-to-lot stability of antibody reagents for flow cytometry. J. Immunol. Methods. 2019;475:112294. doi: 10.1016/j.jim.2017.03.018. - DOI - PubMed
-
- Kim H.S., Kang H.J., Whang D.H., Lee S.G., Park M.J., Park J.Y., Lee K.M. Analysis of reagent lot-to-lot comparability tests in five immunoassay items. Ann. Clin. Lab. Sci. 2012;42:165–173. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

