Imaging of Bioprosthetic Valve Dysfunction after Transcatheter Aortic Valve Implantation
- PMID: 37296760
- PMCID: PMC10253124
- DOI: 10.3390/diagnostics13111908
Imaging of Bioprosthetic Valve Dysfunction after Transcatheter Aortic Valve Implantation
Abstract
Transcatheter aortic valve implantation (TAVI) has become the standard of care in elderly high-risk patients with symptomatic severe aortic stenosis. Recently, TAVI has been increasingly performed in younger-, intermediate- and lower-risk populations, which underlines the need to investigate the long-term durability of bioprosthetic aortic valves. However, diagnosing bioprosthetic valve dysfunction after TAVI is challenging and only limited evidence-based criteria exist to guide therapy. Bioprosthetic valve dysfunction encompasses structural valve deterioration (SVD) resulting from degenerative changes in the valve structure and function, non-SVD resulting from intrinsic paravalvular regurgitation or patient-prosthesis mismatch, valve thrombosis, and infective endocarditis. Overlapping phenotypes, confluent pathologies, and their shared end-stage bioprosthetic valve failure complicate the differentiation of these entities. In this review, we focus on the contemporary and future roles, advantages, and limitations of imaging modalities such as echocardiography, cardiac computed tomography angiography, cardiac magnetic resonance imaging, and positron emission tomography to monitor the integrity of transcatheter heart valves.
Keywords: TAVI; bioprosthetic valve dysfunction; bioprosthetic valve failure; multimodality imaging; structural valve deterioration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

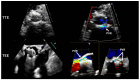


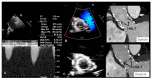
References
-
- Brown J.M., O’Brien S.M., Wu C., Sikora J.A.H., Griffith B.P., Gammie J.S. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J. Thorac. Cardiovasc. Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

