Inverse Propensity Score-Weighted Analysis of Entecavir and Tenofovir Disoproxil Fumarate in Patients with Chronic Hepatitis B: A Large-Scale Multicenter Study
- PMID: 37296898
- PMCID: PMC10252077
- DOI: 10.3390/cancers15112936
Inverse Propensity Score-Weighted Analysis of Entecavir and Tenofovir Disoproxil Fumarate in Patients with Chronic Hepatitis B: A Large-Scale Multicenter Study
Abstract
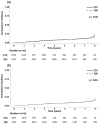
Tenofovir disoproxil fumarate (TDF) is reportedly superior or at least comparable to entecavir (ETV) in preventing hepatocellular carcinoma (HCC) among chronic hepatitis B (CHB) patients; however, it remains controversial. This study aimed to conduct comprehensive comparisons between the two antivirals. CHB patients initially treated with ETV or TDF between 2012 and 2015 at 20 referral centers in Korea were included. The primary outcome was the cumulative incidence of HCC. The secondary outcomes included death or liver transplantation, liver-related outcome, extrahepatic malignancy, development of cirrhosis, decompensation events, complete virologic response (CVR), seroconversion rate, and safety. Baseline characteristics were balanced using the inverse probability of treatment weighting (IPTW). Overall, 4210 patients were enrolled: 1019 received ETV and 3191 received TDF. During the median follow-ups of 5.6 and 5.5 years, 86 and 232 cases of HCC were confirmed in the ETV and TDF groups, respectively. There was no difference in HCC incidence between the groups both before (p = 0.36) and after IPTW was applied (p = 0.81). Although the incidence of extrahepatic malignancy was significantly higher in the ETV group than in the TDF group before weighting (p = 0.02), no difference was confirmed after IPTW (p = 0.29). The cumulative incidence rates of death or liver transplantation, liver-related outcome, new cirrhosis development, and decompensation events were also comparable in the crude population (p = 0.24-0.91) and in the IPTW-adjusted population (p = 0.39-0.80). Both groups exhibited similar rates of CVR (ETV vs. TDF: 95.1% vs. 95.8%, p = 0.38), and negative conversion of hepatitis B e antigen (41.6% vs. 37.2%, p = 0.09) or surface antigen (2.8% vs. 1.9%, p = 0.10). Compared to the ETV group, more patients in the TDF group changed initial antivirals due to side effects, including decreased kidney function (n = 17), hypophosphatemia (n = 20), and osteoporosis (n = 18). In this large-scale multicenter study, ETV and TDF demonstrated comparable effectiveness across a broad range of outcomes in patients with treatment-naïve CHB during similar follow-up periods.
Keywords: entecavir; extrahepatic malignancy; liver cancer; tenofovir; virologic response.
Conflict of interest statement
Yoon Jun Kim receives research grants from BTG, Boston Scientific, AstraZeneca, Gilead Sciences, Samjin, BL&H, and Bayer. Seung Up Kim has served as an advisory committee member of Gilead Sciences, GSK, Bayer, and Eisai. He is a speaker for Gilead Sciences, GSK, Bayer, Eisai, Abbive, EchoSens, MSD, and Bristol-Myers Squibb and has received a research grant from Abbive and Bristol-Myers Squibb. The other authors have nothing to disclose that would be relevant for the publication of this manuscript.
Figures

Similar articles
-
Similar risk of hepatocellular carcinoma during long-term entecavir or tenofovir therapy in Caucasian patients with chronic hepatitis B.J Hepatol. 2020 Nov;73(5):1037-1045. doi: 10.1016/j.jhep.2020.06.011. Epub 2020 Jun 16. J Hepatol. 2020. PMID: 32553667
-
A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea.J Hepatol. 2019 Sep;71(3):456-464. doi: 10.1016/j.jhep.2019.03.028. Epub 2019 Apr 6. J Hepatol. 2019. PMID: 30959156
-
Effectiveness of entecavir vs tenofovir disoproxil fumarate for functional cure of chronic hepatitis B in an international cohort.Hepatol Int. 2022 Dec;16(6):1297-1307. doi: 10.1007/s12072-022-10411-x. Epub 2022 Sep 7. Hepatol Int. 2022. PMID: 36070123
-
Lower risk of hepatocellular carcinoma with tenofovir than entecavir treatment in subsets of chronic hepatitis B patients: an updated meta-analysis.J Gastroenterol Hepatol. 2022 May;37(5):782-794. doi: 10.1111/jgh.15783. Epub 2022 Feb 7. J Gastroenterol Hepatol. 2022. PMID: 35080052 Review.
-
Magnitude of and prediction for risk of hepatocellular carcinoma in patients with chronic hepatitis B taking entecavir or tenofovir therapy: A systematic review.J Gastroenterol Hepatol. 2020 Oct;35(10):1684-1693. doi: 10.1111/jgh.15078. Epub 2020 May 17. J Gastroenterol Hepatol. 2020. PMID: 32343431
Cited by
-
Extrahepatic malignancies and antiviral drugs for chronic hepatitis B: A nationwide cohort study.Clin Mol Hepatol. 2024 Jul;30(3):500-514. doi: 10.3350/cmh.2024.0055. Epub 2024 May 10. Clin Mol Hepatol. 2024. PMID: 38726505 Free PMC article.
References
-
- Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., Chen D.-S., Van Damme P., Abbas Z., Abdulla M., Abou Rached A., Adda D., Aho I., et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

