Calprotectin in Patients with Rheumatic Immunomediated Adverse Effects Induced by Checkpoints Inhibitors
- PMID: 37296947
- PMCID: PMC10251936
- DOI: 10.3390/cancers15112984
Calprotectin in Patients with Rheumatic Immunomediated Adverse Effects Induced by Checkpoints Inhibitors
Abstract
Background: this is an exploratory study to evaluate calprotectin serum levels in patients with rheumatic immune-related adverse events (irAEs) induced by immune checkpoint inhibitor (ICI) treatment.
Methods: this is a retrospective observational study including patients with irAEs rheumatic syndromes. We compared the calprotectin levels to those in a control group of patients with RA and with a control group of healthy individuals. Additionally, we included a control group of patients treated with ICI but without irAEs to check calprotectin levels. We also analysed the performance of calprotectin for the identification of active rheumatic disease using receiver operating characteristic curves (ROC).
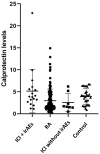
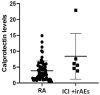
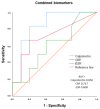
Results: 18 patients with rheumatic irAEs were compared to a control group of 128 RA patients and another group of 29 healthy donors. The mean calprotectin level in the irAE group was 5.15 μg/mL, which was higher than the levels in both the RA group (3.19 μg/mL) and the healthy group (3.81 μg/mL) (cut-off 2 μg/mL). Additionally, 8 oncology patients without irAEs were included. In this group, calprotectin levels were similar to those of the healthy controls. In patients with active inflammation, the calprotectin levels in the irAE group were significantly higher (8.43 μg/mL) compared to the RA group (3.94 μg/mL). ROC curve analysis showed that calprotectin had a very good discriminatory capacity to identify inflammatory activity in patients with rheumatic irAEs (AUC of 0.864).
Conclusions: the results suggest that calprotectin may serve as a marker of inflammatory activity in patients with rheumatic irAEs induced by treatment with ICIs.
Keywords: calprotectin; immune checkpoint inhibitors; immune-related adverse events; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kostine M., Rouxel L., Barnetche T., Veillon R., Martin F., Dutriaux C., Dousset L., Pham-Ledard A., Prey S., Beylot-Barry M., et al. Rheumatic Disorders Associated with Immune Checkpoint Inhibitors in Patients with Cancer-Clinical Aspects and Relationship with Tumour Response: A Single-Centre Prospective Cohort Study. Ann. Rheum. Dis. 2018;77:393–398. doi: 10.1136/annrheumdis-2017-212257. - DOI - PubMed
-
- Mooradian M.J., Nasrallah M., Gainor J.F., Reynolds K.L., Cohen J.V., Lawrence D.P., Miloslavsky E.M., Kohler M.J., Sullivan R.J., Schoenfeld S.R. Musculoskeletal Rheumatic Complications of Immune Checkpoint Inhibitor Therapy: A Single Center Experience. Semin. Arthritis Rheum. 2019;48:1127–1132. doi: 10.1016/j.semarthrit.2018.10.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources

