Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis
- PMID: 37296952
- PMCID: PMC10252039
- DOI: 10.3390/cancers15112987
Matrix Metalloproteinase 9 Induced in Esophageal Squamous Cell Carcinoma Cells via Close Contact with Tumor-Associated Macrophages Contributes to Cancer Progression and Poor Prognosis
Abstract
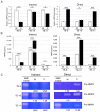
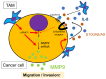
Tumor-associated macrophages (TAMs) contribute to disease progression in various cancers, including esophageal squamous cell carcinoma (ESCC). We have previously used an indirect co-culture system between ESCC cell lines and macrophages to analyze their interactions. Recently, we established a direct co-culture system to closely simulate actual ESCC cell-TAM contact. We found that matrix metalloproteinase 9 (MMP9) was induced in ESCC cells by direct co-culture with TAMs, not by indirect co-culture. MMP9 was associated with ESCC cell migration and invasion, and its expression was controlled by the Stat3 signaling pathway in vitro. Immunohistochemical analyses revealed that MMP9 expression in cancer cells at the invasive front ("cancer cell MMP9") was related to high infiltration of CD204 positive M2-like TAMs (p < 0.001) and was associated with worse overall and disease-free survival of patients (p = 0.036 and p = 0.038, respectively). Furthermore, cancer cell MMP9 was an independent prognostic factor for disease-free survival. Notably, MMP9 expression in cancer stroma was not associated with any clinicopathological factors or patient prognoses. Our results suggest that close interaction with TAMs infiltrating in cancer stroma or cancer nests induces MMP9 expression in ESCC cells, equipping them with more malignant features.
Keywords: activator of transcription 3; direct co-culture; direct contact; esophageal squamous cell carcinoma; immunohistochemistry; interleukin-8; matrix metalloproteinase 9; prognostic factor; signal transducer; tumor-associated macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cancer Statistics Cancer Information Service, National Cancer Center, Japan (National Cancer Registry, Ministry of Health, Labour and Welfare) [(accessed on 24 February 2023)]. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#a14.
-
- Shimada H., Nabeya Y., Okazumi S.-I., Matsubara H., Shiratori T., Gunji Y., Kobayashi S., Hayashi H., Ochiai T. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery. 2003;133:486–494. doi: 10.1067/msy.2003.139. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

