The Structural Dynamics, Complexity of Interactions, and Functions in Cancer of Multi-SAM Containing Proteins
- PMID: 37296980
- PMCID: PMC10252437
- DOI: 10.3390/cancers15113019
The Structural Dynamics, Complexity of Interactions, and Functions in Cancer of Multi-SAM Containing Proteins
Abstract
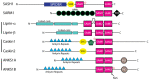
SAM domains are crucial mediators of diverse interactions, including those important for tumorigenesis or metastasis of cancers, and thus SAM domains can be attractive targets for developing cancer therapies. This review aims to explore the literature, especially on the recent findings of the structural dynamics, regulation, and functions of SAM domains in proteins containing more than one SAM (multi-SAM containing proteins, MSCPs). The topics here include how intrinsic disorder of some SAMs and an additional SAM domain in MSCPs increase the complexity of their interactions and oligomerization arrangements. Many similarities exist among these MSCPs, including their effects on cancer cell adhesion, migration, and metastasis. In addition, they are all involved in some types of receptor-mediated signaling and neurology-related functions or diseases, although the specific receptors and functions vary. This review also provides a simple outline of methods for studying protein domains, which may help non-structural biologists to reach out and build new collaborations to study their favorite protein domains/regions. Overall, this review aims to provide representative examples of various scenarios that may provide clues to better understand the roles of SAM domains and MSCPs in cancer in general.
Keywords: CASKIN; Eph receptors; LAR-RPTP; Liprin; SASH1; cancer; disordered SAM domains; metastasis; tumor suppression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cancer. [(accessed on 26 April 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

