Biocompatibility, Surface Morphology, and Bacterial Load of Dental Implant Abutments following Decontamination Protocols: An In-Vitro Study
- PMID: 37297212
- PMCID: PMC10254387
- DOI: 10.3390/ma16114080
Biocompatibility, Surface Morphology, and Bacterial Load of Dental Implant Abutments following Decontamination Protocols: An In-Vitro Study
Abstract
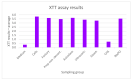
The long-term success of dental implant rehabilitation depends significantly on proper peri-implant soft tissue integration. Therefore, decontamination of abutments prior to their connection to the implant is beneficial to enhance soft tissue attachment and to aid in maintaining marginal bone around the implant. Consequently, different implant abutment decontamination protocols were evaluated regarding biocompatibility, surface morphology, and bacterial load. The protocols evaluated were autoclave sterilization, ultrasonic washing, steam cleaning, chlorhexidine chemical decontamination, and sodium hypochlorite chemical decontamination. The control groups included: (1) implant abutments prepared and polished in a dental lab without decontamination and (2) unprepared implant abutments obtained directly from the company. Surface analysis was performed using scanning electron microscopy (SEM). Biocompatibility was evaluated using XTT cell viability and proliferation assays. Biofilm biomass and viable counts (CFU/mL) (n = 5 for each test) were used for surface bacterial load evaluation. Surface analysis revealed areas of debris and accumulation of materials, such as iron, cobalt, chromium, and other metals, in all abutments prepared by the lab and with all decontamination protocols. Steam cleaning was the most efficient method for reducing contamination. Chlorhexidine and sodium hypochlorite left residual materials on the abutments. XTT results showed that the chlorhexidine group (M = 0.7005, SD = 0.2995) had the lowest values (p < 0.001) (autoclave: M = 3.6354, SD = 0.1510; ultrasonic: M = 3.4077, SD = 0.3730; steam: M = 3.2903, SD = 0.2172; NaOCl: M = 3.5377, SD = 0.0927; prep non-decont.: M = 3.4815, SD = 0.2326; factory: M = 3.6173, SD = 0.0392). Bacterial growth (CFU/mL) was high in the abutments treated with steam cleaning and ultrasonic bath: 2.93 × 109, SD = 1.68 × 1012 and 1.83 × 109, SD = 3.95 × 1010, respectively. Abutments treated with chlorhexidine showed higher toxicity to cells, while all other samples showed similar effects to the control. In conclusion, steam cleaning seemed to be the most efficient method for reducing debris and metallic contamination. Bacterial load can be reduced using autoclaving, chlorhexidine, and NaOCl.
Keywords: decontamination; osseointegration; peri-implantitis; steam; titanium; ultrasonics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Effect of chemical and electrochemical decontamination protocols on single and multiple-used healing abutments: A comparative analysis of contact surface area, micro-gap, micro-leakage, and surface topography.Clin Implant Dent Relat Res. 2023 Dec;25(6):1207-1215. doi: 10.1111/cid.13269. Epub 2023 Sep 1. Clin Implant Dent Relat Res. 2023. PMID: 37654160 Clinical Trial.
-
Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures.Clin Oral Implants Res. 2014 Mar;25(3):328-336. doi: 10.1111/clr.12089. Epub 2012 Dec 5. Clin Oral Implants Res. 2014. PMID: 23210704
-
Fibrin clot adherence on cleaned and decontaminated titanium abutment surfaces: An in vitro study.Clin Implant Dent Relat Res. 2024 Dec;26(6):1190-1199. doi: 10.1111/cid.13366. Epub 2024 Aug 29. Clin Implant Dent Relat Res. 2024. PMID: 39210678
-
Dental implant healing abutment decontamination: A systematic review of in vitro studies.Int J Oral Implantol (Berl). 2022 Nov 15;15(4):311-324. Int J Oral Implantol (Berl). 2022. PMID: 36377623
-
Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review.Int J Oral Maxillofac Implants. 2012 Sep-Oct;27(5):1043-54. Int J Oral Maxillofac Implants. 2012. PMID: 23057016 Review.
References
-
- Pjetursson B.E., Sailer I., Latyshev A., Rabel K., Kohal R.J., Karasan D. A systematic review and meta-analysis evaluating the survival, the failure, and the complication rates of veneered and monolithic all-ceramic implant-supported single crowns. Clin. Oral Implants Res. 2021;32((Suppl. S21)):254–288. doi: 10.1111/clr.13863. - DOI - PMC - PubMed
-
- Pjetursson B.E., Thoma D., Jung R., Zwahlen M., Zembic A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012;23((Suppl. S6)):22–38. doi: 10.1111/j.1600-0501.2012.02546.x. - DOI - PubMed
-
- Jung R.E., Zembic A., Pjetursson B.E., Zwahlen M., Thoma D.S. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implants Res. 2012;23((Suppl. S6)):2–21. doi: 10.1111/j.1600-0501.2012.02547.x. - DOI - PubMed
-
- Welander M., Abrahamsson I., Berglundh T. The mucosal barrier at implant abutments of different materials. Clin. Oral Implants Res. 2008;19:635–641. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

