Exploration of Optimal pH in Hypothermic Machine Perfusion for Rat Liver Grafts Retrieved after Circulatory Death
- PMID: 37298042
- PMCID: PMC10253457
- DOI: 10.3390/jcm12113845
Exploration of Optimal pH in Hypothermic Machine Perfusion for Rat Liver Grafts Retrieved after Circulatory Death
Abstract
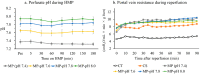
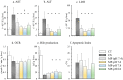
Ex vivo hypothermic machine perfusion (HMP) is a strategy for controlling ischemia-reperfusion injury in donation after circulatory death (DCD) liver transplantation. The pH of blood increases with a decrease in temperature and water dissociation, leading to a decrease in [H+]. This study aimed to verify the optimal pH of HMP for DCD livers. Rat livers were retrieved 30 min post-cardiac arrest and subjected to 3-h cold storage (CS) in UW solution (CS group) or HMP with UW-gluconate solution (machine perfusion [MP] group) of pH 7.4 (original), 7.6, 7.8, and 8.0 (MP-pH 7.6, 7.8, 8.0 groups, respectively) at 7-10 °C. The livers were subjected to normothermic perfusion to simulate reperfusion after HMP. All HMP groups showed greater graft protection compared to the CS group due to the lower levels of liver enzymes in the former. The MP-pH 7.8 group showed significant protection, evidenced by bile production, diminished tissue injury, and reduced flavin mononucleotide leakage, and further analysis by scanning electron microscopy revealed a well-preserved structure of the mitochondrial cristae. Therefore, the optimum pH of 7.8 enhanced the protective effect of HMP by preserving the structure and function of the mitochondria, leading to reduced reperfusion injury in the DCD liver.
Keywords: DCD; donation after circulatory death; hypothermic machine perfusion; liver; machine perfusion; mitochondria; osmium-maceration; pH; transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

