Microglia and the Blood-Brain Barrier: An External Player in Acute and Chronic Neuroinflammatory Conditions
- PMID: 37298096
- PMCID: PMC10252410
- DOI: 10.3390/ijms24119144
Microglia and the Blood-Brain Barrier: An External Player in Acute and Chronic Neuroinflammatory Conditions
Abstract
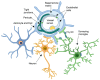
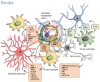
Microglia are the resident immune cells of the central nervous system that guarantee immune surveillance and exert also a modulating role on neuronal synaptic development and function. Upon injury, microglia get activated and modify their morphology acquiring an ameboid phenotype and pro- or anti-inflammatory features. The active role of microglia in blood-brain barrier (BBB) function and their interaction with different cellular components of the BBB-endothelial cells, astrocytes and pericytes-are described. Here, we report the specific crosstalk of microglia with all the BBB cell types focusing in particular on the involvement of microglia in the modulation of BBB function in neuroinflammatory conditions that occur in conjunction with an acute event, such as a stroke, or in a slow neurodegenerative disease, such as Alzheimer's disease. The potential of microglia to exert a dual role, either protective or detrimental, depending on disease stages and environmental conditioning factors is also discussed.
Keywords: Alzheimer’s disease; BBB; astrocyte; endothelial cells; microglia; stroke.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

