Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor-From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety
- PMID: 37298103
- PMCID: PMC10252989
- DOI: 10.3390/ijms24119152
Significance of Astragaloside IV from the Roots of Astragalus mongholicus as an Acetylcholinesterase Inhibitor-From the Computational and Biomimetic Analyses to the In Vitro and In Vivo Studies of Safety
Abstract
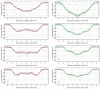
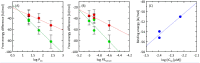
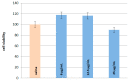
The main aim of the study was to assess the acetylcholinesterase-inhibitory potential of triterpenoid saponins (astragalosides) found in the roots of Astragalus mongholicus. For this purpose, the TLC bioautography method was applied and then the IC50 values were calculated for astragalosides II, III and IV (5.9 μM; 4.2 μM, and 4.0 μM, respectively). Moreover, molecular dynamics simulations were carried outto assess the affinity of the tested compounds for POPC and POPG-containing lipid bilayers, which in this case are the models of the blood-brain barrier (BBB). All determined free energy profiles confirmed that astragalosides exhibit great affinity for the lipid bilayer. A good correlation was obtained when comparing the logarithm of n-octanol/water partition coefficient (logPow) lipophilicity descriptor values with the smallest values of free energy of the determined 1D profiles. The affinity for the lipid bilayers changes in the same order as the corresponding logPow values, i.e.,: I > II > III~IV. All compounds exhibit a high and also relatively similar magnitude of binding energies, varying from ca. -55 to -51 kJ/mol. Apositive correlation between the experimentally-determined IC50 values and the theoretically-predicted binding energies expressed by the correlation coefficient value equal 0.956 was observed.
Keywords: IC50; SH-SY5Y; acetylcholinesterase; free energy; lipophilicity; molecular docking; safety; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
In Silico Studies on Triterpenoid Saponins Permeation through the Blood-Brain Barrier Combined with Postmortem Research on the Brain Tissues of Mice Affected by Astragaloside IV Administration.Int J Mol Sci. 2020 Apr 5;21(7):2534. doi: 10.3390/ijms21072534. Int J Mol Sci. 2020. PMID: 32260588 Free PMC article.
-
Accumulation of astragalosides and related gene expression in different organs of Astragalus membranaceus Bge. var mongholicus (Bge.).Molecules. 2014 Jul 25;19(8):10922-35. doi: 10.3390/molecules190810922. Molecules. 2014. PMID: 25068786 Free PMC article.
-
Astragaloside content in the periderm, cortex, and xylem of Astragalus membranaceus root.J Nat Med. 2013 Oct;67(4):850-5. doi: 10.1007/s11418-013-0741-8. Epub 2013 Feb 5. J Nat Med. 2013. PMID: 23381847
-
Research progress of natural medicine Astragalus mongholicus Bunge in treatment of myocardial fibrosis.J Ethnopharmacol. 2023 Apr 6;305:116128. doi: 10.1016/j.jep.2022.116128. Epub 2023 Jan 6. J Ethnopharmacol. 2023. PMID: 36623754 Review.
-
Pharmacological effects of Astragaloside IV: a literature review.J Tradit Chin Med. 2013 Jun;33(3):413-6. doi: 10.1016/s0254-6272(13)60189-2. J Tradit Chin Med. 2013. PMID: 24024343 Review.
Cited by
-
Astragaloside IV as a Memory-Enhancing Agent: In Silico Studies with In Vivo Analysis and Post Mortem ADME-Tox Profiling in Mice.Int J Mol Sci. 2024 Apr 4;25(7):4021. doi: 10.3390/ijms25074021. Int J Mol Sci. 2024. PMID: 38612831 Free PMC article.
-
Neuroprotective Properties of Oleanolic Acid-Computational-Driven Molecular Research Combined with In Vitro and In Vivo Experiments.Pharmaceuticals (Basel). 2023 Aug 31;16(9):1234. doi: 10.3390/ph16091234. Pharmaceuticals (Basel). 2023. PMID: 37765042 Free PMC article.
-
In Silico Analysis, Anticonvulsant Activity, and Toxicity Evaluation of Schisandrin B in Zebrafish Larvae and Mice.Int J Mol Sci. 2023 Aug 18;24(16):12949. doi: 10.3390/ijms241612949. Int J Mol Sci. 2023. PMID: 37629132 Free PMC article.
-
Characterization of Phenolic Profile and Biological Properties of Astragalus membranaceus Fisch. ex Bunge Commercial Samples.Antioxidants (Basel). 2024 Aug 16;13(8):993. doi: 10.3390/antiox13080993. Antioxidants (Basel). 2024. PMID: 39199238 Free PMC article.
-
In Vivo Insights into the Role of Astragaloside IV in Preventing and Treating Civilization Diseases: A Comprehensive Review.Int J Mol Sci. 2025 Apr 29;26(9):4250. doi: 10.3390/ijms26094250. Int J Mol Sci. 2025. PMID: 40362487 Free PMC article. Review.
References
-
- Quinn D.M. Acetylcholinesterase: Enzyme structure reaction dynamics and virtual transition states. Chem. Rev. 1987;87:955–979. doi: 10.1021/cr00081a005. - DOI
-
- Chang Y., Bai M., Zhang X., Shen S., Hou J.-Y., Yao G.-D., Huang X.-X., Song S.J. Neuroprotective and acetylcholinesterase inhibitory activities of alkaloids from Solanum lyratum Thunb.: An in vitro and in silico analyses. Phytochemistry. 2023;209:113623. doi: 10.1016/j.phytochem.2023.113623. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

