Entangled Motifs in Membrane Protein Structures
- PMID: 37298146
- PMCID: PMC10253074
- DOI: 10.3390/ijms24119193
Entangled Motifs in Membrane Protein Structures
Abstract
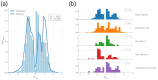
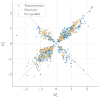
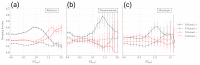
Entangled motifs are found in one-third of protein domain structures, a reference set that contains mostly globular proteins. Their properties suggest a connection with co-translational folding. Here, we wish to investigate the presence and properties of entangled motifs in membrane protein structures. From existing databases, we build a non-redundant data set of membrane protein domains, annotated with the monotopic/transmembrane and peripheral/integral labels. We evaluate the presence of entangled motifs using the Gaussian entanglement indicator. We find that entangled motifs appear in one-fifth of transmembrane and one-fourth of monotopic proteins. Surprisingly, the main features of the distribution of the values of the entanglement indicator are similar to the reference case of general proteins. The distribution is conserved across different organisms. Differences with respect to the reference set emerge when considering the chirality of entangled motifs. Although the same chirality bias is found for single-winding motifs in both membrane and reference proteins, the bias is reversed, strikingly, for double-winding motifs only in the reference set. We speculate that these observations can be rationalized in terms of the constraints exerted on the nascent chain by the co-translational bio-genesis machinery, which is different for membrane and globular proteins.
Keywords: chirality; co-translational folding; entanglement; membrane proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

