Procoagulant Activity of Umbilical Cord-Derived Mesenchymal Stromal Cells' Extracellular Vesicles (MSC-EVs)
- PMID: 37298168
- PMCID: PMC10252357
- DOI: 10.3390/ijms24119216
Procoagulant Activity of Umbilical Cord-Derived Mesenchymal Stromal Cells' Extracellular Vesicles (MSC-EVs)
Abstract
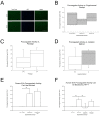
Many cell types, including cancer cells, release tissue factor (TF)-exposing extracellular vesicles (EVs). It is unknown whether MSC-EVs pose a thromboembolism risk due to TF expression. Knowing that MSCs express TF and are procoagulant, we hypothesize that MSC-EVs also might. Here, we examined the expression of TF and the procoagulant activity of MSC-EVs and the impact of EV isolation methods and cell culture expansion on EV yield, characterization, and potential risk using a design of experiments methodology. MSC-EVs were found to express TF and have procoagulant activity. Thus, when MSC-derived EVs are employed as a therapeutic agent, one might consider TF, procoagulant activity, and thromboembolism risk and take steps to prevent them.
Keywords: canine; clinical safety; exosome; hemocompatibility; human; mesenchymal stromal cells; tissue factor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

