The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1
- PMID: 37298172
- PMCID: PMC10252925
- DOI: 10.3390/ijms24119221
The Effect of Statins on Male Reproductive Parameters: A Mechanism Involving Dysregulation of Gonadal Hormone Receptors and TRPV1
Abstract
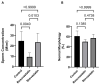
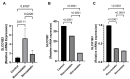
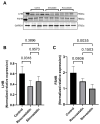
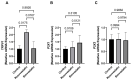
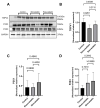
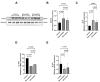
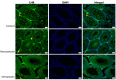
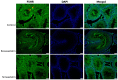
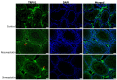
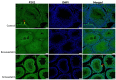
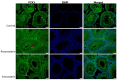
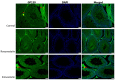
Statins have been shown to cause diverse male reproductive function impairment, and in some cases, orchialgia. Therefore, the current study investigated the possible mechanisms through which statins may alter male reproductive parameters. Thirty adult male Wistar rats (200-250 g) were divided into three groups. The animals were orally administered rosuvastatin (50 mg/kg), simvastatin (50 mg/kg), or 0.5% carboxy methyl cellulose (control), for a 30-day period. Spermatozoa were retrieved from the caudal epididymis for sperm analysis. The testis was used for all biochemical assays and immunofluorescent localization of biomarkers of interest. Rosuvastatin-treated animals presented with a significant decrease in sperm concentration when compared to both the control and simvastatin groups (p < 0.005). While no significant difference was observed between the simvastatin and the control group. The Sertoli cells, Leydig cells and whole testicular tissue homogenate expressed transcripts of solute carrier organic anion transporters (SLCO1B1 and SLCO1B3). There was a significant decrease in the testicular protein expression of the luteinizing hormone receptor, follicle stimulating hormone receptor, and transient receptor potential vanilloid 1 in the rosuvastatin and simvastatin-treated animals compared to the control. The expression of SLCO1B1, SLCO1B2, and SLCO1B3 in the different spermatogenic cells portray that un-bio transformed statin can be transported into the testicular microenvironment, which can subsequently alter the regulation of the gonadal hormone receptors, dysregulate pain-inflammatory biomarkers, and consequently impair sperm concentration.
Keywords: inflammation; male infertility; rosuvastatin; sex hormone receptors; simvastatin; testicular pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Navarese E.P., Buffon A., Andreotti F., Kozinski M., Welton N., Fabiszak T., Caputo S., Grzesk G., Kubica A., Swiatkiewicz I., et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am. J. Cardiol. 2013;111:1123–1130. doi: 10.1016/j.amjcard.2012.12.037. - DOI - PubMed
-
- Leite G.A.A., de lima Rosa J., Sanabria M., Cavariani M.M., Franci J.A.A., Pinheiro P.F.F., Kempinas W.D.G. Delayed reproductive development in pubertal male rats exposed to the hypolipemiant agent rosuvastatin since prepuberty. Reprod. Toxicol. 2014;44:93–103. doi: 10.1016/j.reprotox.2014.01.004. - DOI - PubMed
-
- Leite G.A.A., de Barros J.W.F., de Martins A.C., Anselmo-Franci J.A., Barbosa F., Kempinas W.D.G. Ascorbic acid supplementation ameliorates testicular hormonal signaling, sperm production and oxidative stress in male rats exposed to rosuvastatin during pre-puberty. J. Appl. Toxicol. 2019;39:305–321. doi: 10.1002/jat.3720. - DOI - PubMed
-
- E Silva P.V., Borges C.D.S., Rosa J.D.L., Pacheco T.L., Figueiredo T.M., Leite G.A.A., Guerra M.T., Anselmo-Franci J.A., Klinefelter G.R., Kempinas W.D.G. Effects of isolated or combined exposure to sibutramine and rosuvastatin on reproductive parameters of adult male rats. J. Appl. Toxicol. 2020;40:947–964. doi: 10.1002/jat.3955. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

